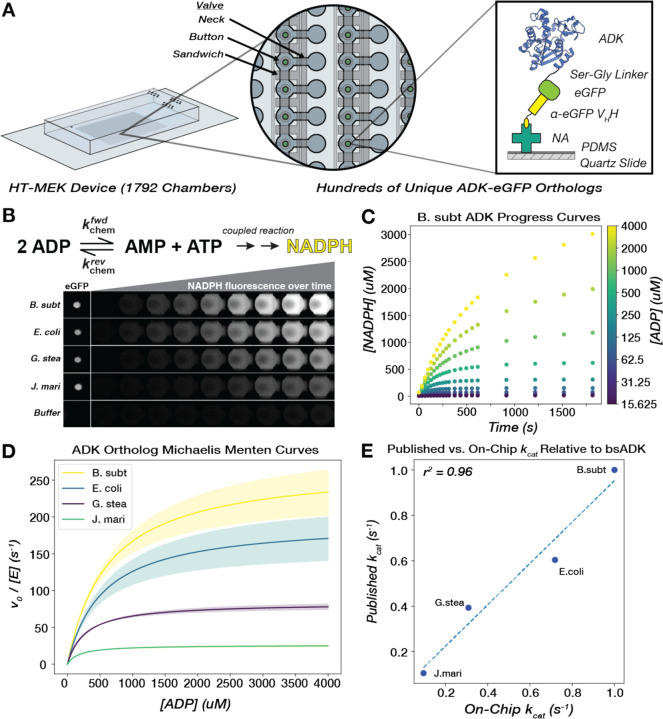
Figure 2. Michaelis-Menten parameters for hundreds of naturally occurring ADK sequences can be measured in parallel via high-throughput microfluidic devices.
(A) A single High-Throughput Microfluidic Enzyme Kinetics (HT-MEK) device enabled the expression and purification of up to 1792 enzyme variants in a single experiment. All enzyme variants are tagged with a C-terminal eGFP construct to facilitate capture on a functionalized "pedestal." This pedestal consists of neutravidin proteins (NA) non-specifically bound to a PDMS-coated quartz slide, which in turn binds a biotinylated anti-eGFP VHH nanobody, pulling down the eGFP-tagged ADK in each chamber. (B) ADK activity in the direction of ATP formation is monitored on-chip through coupled production of NADPH, and product formation is measured over the course of the assay with time-lapse inverted fluorescent microscopy. Four chambers containing exemplary orthologs are highlighted, as well as a control chamber that did not contain an ADK-encoding plasmid and thus did not show any expressed enzyme or detectable catalysis. (C) Scatter plot of progress curves for bsADK across multiple substrate concentrations (encoded by color). (D) Mean fits of initial rates to the Michaelis-Menten equation for four ADK orthologs. Shaded regions represent the standard deviation of kcat across biological replicates for each ortholog. (E) On-chip catalytic measurements correlate with previously published values for orthologs, relative to bsADK, measured in the same reaction direction under comparable conditions (Methods).