Figure 4. Anticipated and deduced molecular mechanisms of gB fusion.
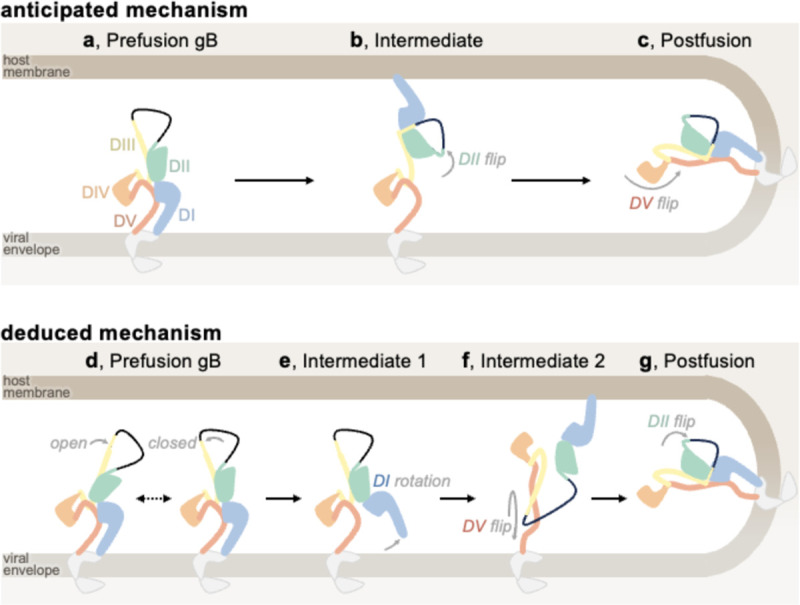
Domain I (DI, blue), domain II (DII, green), domain III (DIII, yellow), domain IV (DIV, orange), and domain V (DV, red) are shown, in addition to the loop between DII and DIII (black line), cytoplasmic and transmembrane portions of gB (grey), the viral envelope (light brown), and host membrane (dark brown). (a-c) Cartoon schematic of the anticipated mechanism of gB-mediated fusion at the outset of the study: starting from prefusion gB (a), DII flips about DIII, positioning DI to bind the host membrane, (b), and then DV flips about DIII, yielding the postfusion conformation while merging viral and host membranes (c). (d-g) The mechanism of gB-mediated fusion which is consistent with the analysis of gB ectodomains herein: prefusion gB DIII may exist in open and/or closed conformations, likely depending on the virus (d). The hinge between DI and DII restructures leading to DI rotation; thereby pulling the fusion loops from the viral envelope while releasing DV (e). DV refolds to its postfusion conformation and replaces DII on DIII, releasing DII. Since DV is anchored to the viral envelope, this conformational change reorients the entire ectodomain and positions the DI fusion loops for binding the host membrane (f). DII binds its postfusion interface on DIII, yielding the postfusion gB conformation (g).
