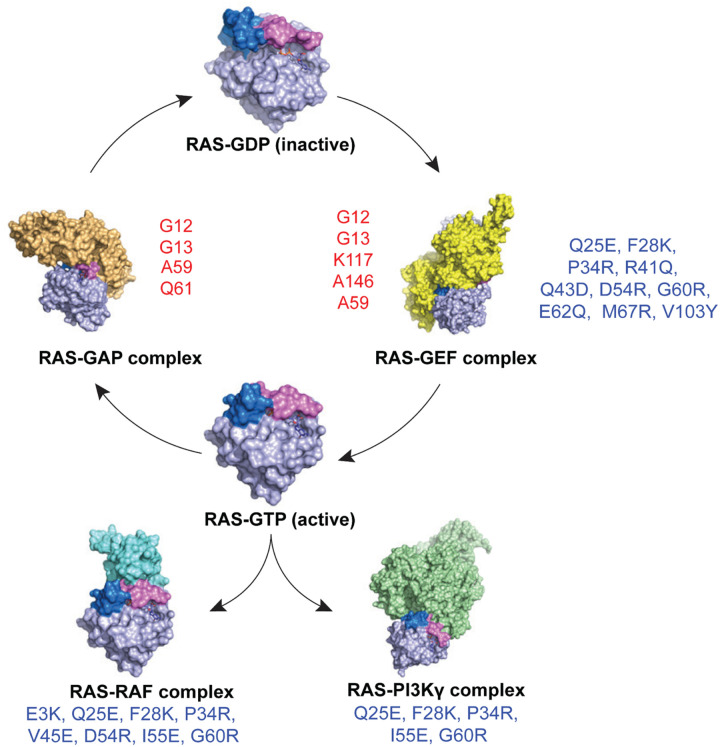
Figure 5. Schematic representation illustrating the impact of second-site inactivating mutations of KRASG12D.
Schematic representation of KRAS cycling between its inactive GDP-bound form (RAS-GDP) and active GTP-bound form (RAS-GTP), as well as its interactions with regulatory proteins and effectors. The RAS-GDP state is shown at the top center, transitioning to the RAS-GEF complex (right), which facilitates nucleotide exchange. The GTP-bound RAS engages with downstream effectors, including RAS-RAF and RAS-PI3K. RAS-GAP inactivates RAS by promoting GTP hydrolysis, returning RAS to its GDP-bound state. KRAS gain-of-function mutations (red - G12, G13, A59, K117, A146, and Q61) lead to constitutive activation of RAS. Loss-of-function mutations (blue – E3, Q25, F28, P34, R41, Q43, V45, D54, I55, G60, E62, M67, and V103) disrupt interactions with effectors and regulatory proteins (RasGEFs), resulting in reduced oncogenicity of KRASG12D.