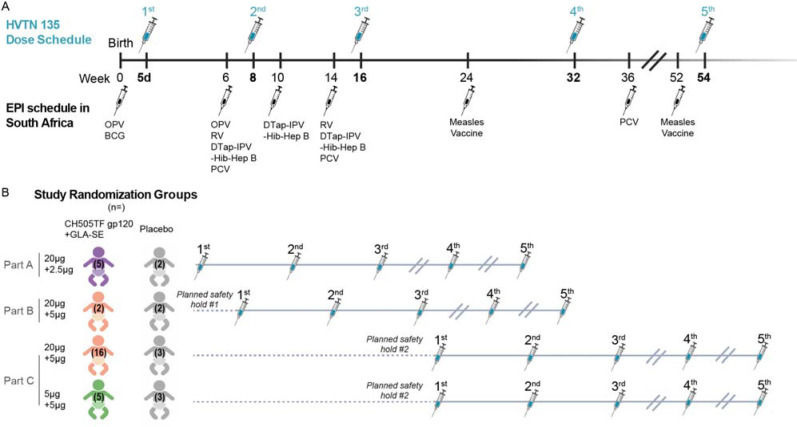
Figure 2. Study design.
(A) Vaccine dosing schedule in relation to EPI vaccinations. (B) Study Schema: Healthy infants without HIV born to mothers living with HIV were randomized to receive 5 doses of CH505TF gp120 + GLA-SE vaccine or placebo, with the first dose within 5 days of birth. The 3-part design allowed for an initial assessment of safety in Part A before the target adjuvant dose was studied in Part B. Part C compared two different doses of CH505TF gp120 while keeping the same adjuvant dose.