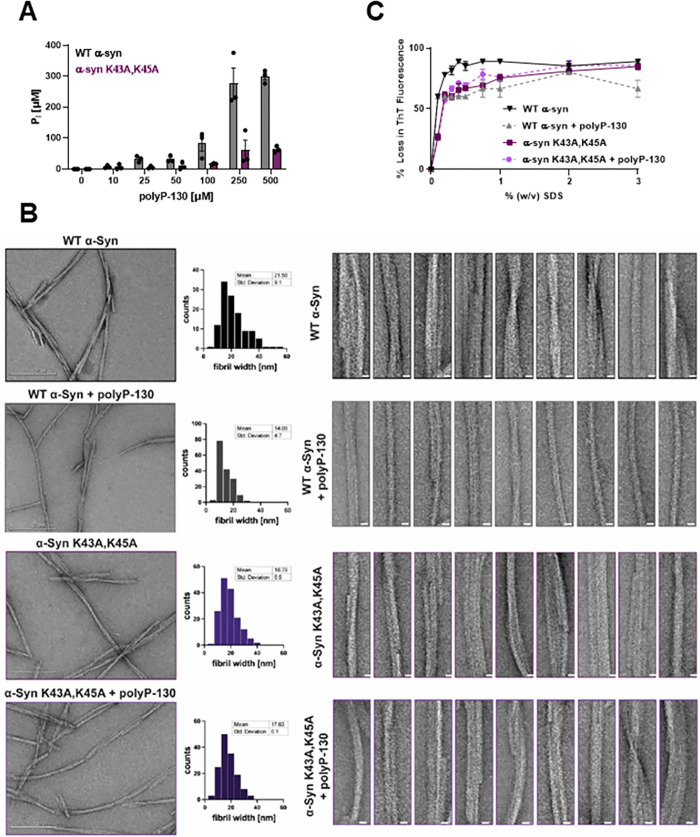
Fig 3. Effects of K43, K45 substitution on polyP binding, fibril stability and morphology.
(A) Fibrils of 100 μM WT α-Syn or α-SynK43A,45A were formed in the absence or presence of increasing amounts of polyP-130 for 48 h. Fibrils were pelleted, washed with high salt buffer and digested in 1 M hydrochloric acid to hydrolyze all bound polyP. Free Pi was measured using molybdate. (B) TEM of 100 μM WT α-Syn or α-SynK43A,K45A fibrils formed in the absence or presence of 500 μM polyP-130. Frequency distribution plots of the fibril width distributions and the mean fibril width and standard deviation are given in the middle panel. Ten representative filaments are presented on the right for each condition (scale bar is 10 nm). At least 100 fibrils per condition were measured across 3 separate experiments. (C) Fibrils were prepared in the absence or presence of 500 μM polyP-130 as before. Fibrils of WT α-Syn (black/gray) or α-SynK43A,K45A mutant (light and dark purple) fibrils were supplemented with ThT and incubated with increasing concentrations of SDS at 37°C for 5 min. Changes in ThT fluorescence were recorded; n = 3; mean ± SD is shown. The underlying data can be found in Mendeley (see data statement for details).