Abstract
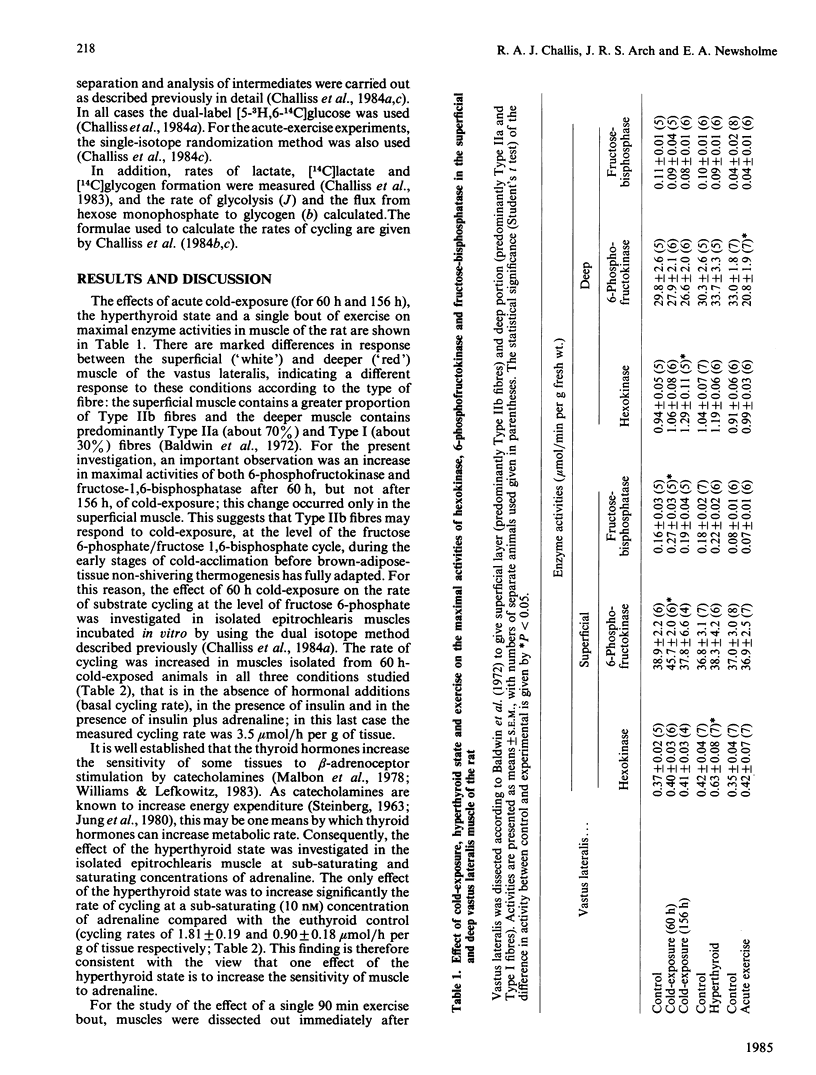
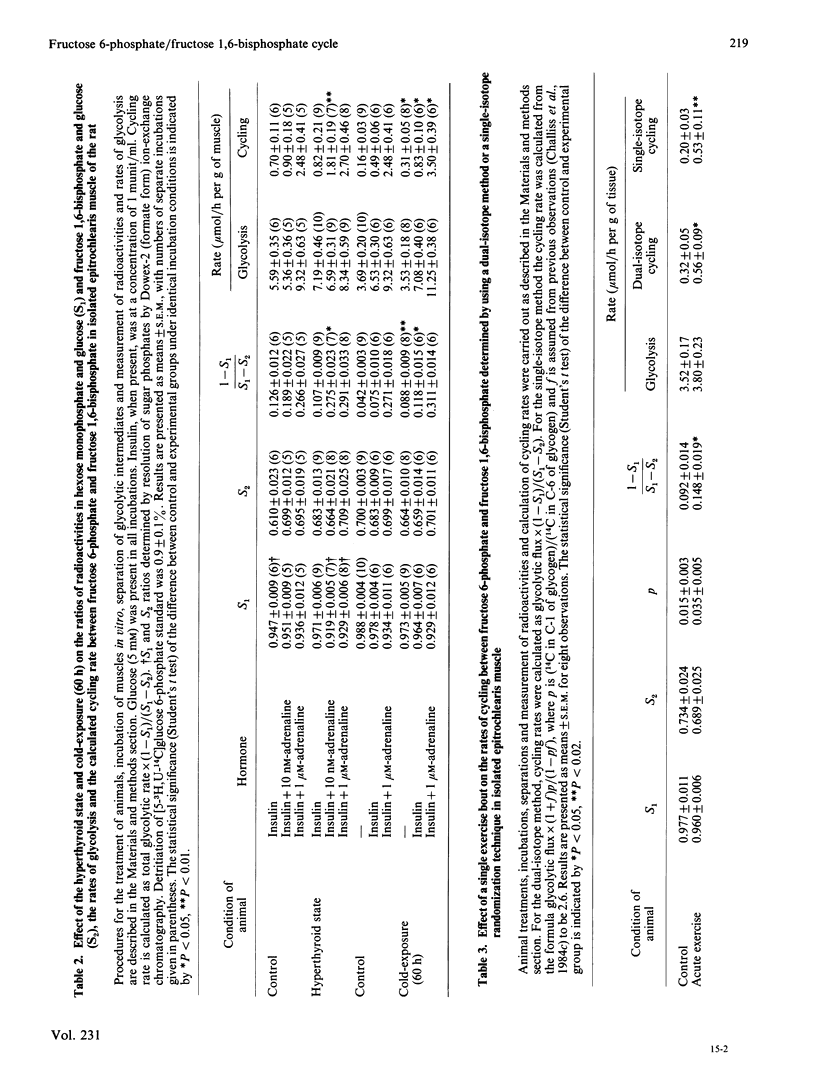
The effects of cold-exposure, the hyperthyroid state and a single exercise bout in vivo on the maximal enzyme activities of 6-phosphofructokinase and fructose-1,6-bisphosphatase in vastus lateralis muscle and the rates of fructose 6-phosphate/fructose 1,6-bisphosphate cycling measured in epitrochlearis muscle in vitro were investigated. In all cases significant changes in substrate cycling rates were observed, whether in the absence of added hormones in vitro (acute exercise), or when stimulated by insulin plus adrenaline (cold-exposure), or with respect to the catecholamine-sensitivity of the cycling rate (the hyperthyroid state).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin K. M., Klinkerfuss G. H., Terjung R. L., Molé P. A., Holloszy J. O. Respiratory capacity of white, red, and intermediate muscle: adaptative response to exercise. Am J Physiol. 1972 Feb;222(2):373–378. doi: 10.1152/ajplegacy.1972.222.2.373. [DOI] [PubMed] [Google Scholar]
- Brooks B. J., Arch J. R., Newsholme E. A. Effect of some hormones on the rate of the triacylglycerol/fatty-acid substrate cycle in adipose tissue of the mouse in vivo. Biosci Rep. 1983 Mar;3(3):263–267. doi: 10.1007/BF01122458. [DOI] [PubMed] [Google Scholar]
- Challiss R. A., Arch J. R., Crabtree B., Newsholme E. A. Measurement of the rate of substrate cycling between fructose 6-phosphate and fructose 1,6-bisphosphate in skeletal muscle by using a single-isotope technique. Biochem J. 1984 Nov 1;223(3):849–853. doi: 10.1042/bj2230849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challiss R. A., Arch J. R., Newsholme E. A. The rate of substrate cycling between fructose 6-phosphate and fructose 1,6-bisphosphate in skeletal muscle. Biochem J. 1984 Jul 1;221(1):153–161. doi: 10.1042/bj2210153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challiss R. A., Crabtree B., Newsholme E. A. Appendix: Calculation of the rate of fructose 6-phosphate/fructose 1,6-bisphosphate cycling in a tissue with active glycogenolysis and/or glycogen synthesis. Biochem J. 1984 Jul 1;221(1):160–161. doi: 10.1042/bj2210160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challiss R. A., Espinal J., Newsholme E. A. Insulin sensitivity of rates of glycolysis and glycogen synthesis in soleus, stripped soleus, epitrochlearis, and hemi-diaphragm muscles isolated from sedentary rats. Biosci Rep. 1983 Jul;3(7):675–679. doi: 10.1007/BF01172878. [DOI] [PubMed] [Google Scholar]
- Crabtree B., Higgins S. J., Newsholme E. A. The activities of pyruvate carboxylase, phosphoenolpyruvate carboxylase and fructose diphosphatase in muscles from vertebrates and invertebrates. Biochem J. 1972 Nov;130(2):391–396. doi: 10.1042/bj1300391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohm G. L., Tapscott E. B., Barakat H. A., Kasperek G. J. Influence of fasting on glycogen depletion in rats during exercise. J Appl Physiol Respir Environ Exerc Physiol. 1983 Sep;55(3):830–833. doi: 10.1152/jappl.1983.55.3.830. [DOI] [PubMed] [Google Scholar]
- Gleeson M., Brown J. F., Waring J. J., Stock M. J. The effects of physical exercise on metabolic rate and dietary-induced thermogenesis. Br J Nutr. 1982 Mar;47(2):173–181. doi: 10.1079/bjn19820025. [DOI] [PubMed] [Google Scholar]
- Huang M. T., Lardy H. A. Effects of thyroid states on the Cori cycle, glucose--alanine cycle, and futile cycling of glucose metabolism in rats. Arch Biochem Biophys. 1981 Jun;209(1):41–51. doi: 10.1016/0003-9861(81)90254-x. [DOI] [PubMed] [Google Scholar]
- Jung R. T., Shetty P. S., James W. P. Nutritional effects on thyroid and catecholamine metabolism. Clin Sci (Lond) 1980 Mar;58(3):183–191. doi: 10.1042/cs0580183. [DOI] [PubMed] [Google Scholar]
- Malbon C. C., Moreno F. J., Cabelli R. J., Fain J. N. Fat cell adenylate cyclase and beta-adrenergic receptors in altered thyroid states. J Biol Chem. 1978 Feb 10;253(3):671–678. [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B. Metabolic aspects of enzyme activity regulation. Symp Soc Exp Biol. 1973;27:429–460. [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B. Substrate cycles in metabolic regulation and in heat generation. Biochem Soc Symp. 1976;(41):61–109. [PubMed] [Google Scholar]
- Newsholme E. A. Sounding Board. A possible metabolic basis for the control of body weight. N Engl J Med. 1980 Feb 14;302(7):400–405. doi: 10.1056/NEJM198002143020711. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A. Substrate cycles: their metabolic, energetic and thermic consequences in man. Biochem Soc Symp. 1978;(43):183–205. [PubMed] [Google Scholar]
- Opie L. H., Newsholme E. A. The activities of fructose 1,6-diphosphatase, phosphofructokinase and phosphoenolpyruvate carboxykinase in white muscle and red muscle. Biochem J. 1967 May;103(2):391–399. doi: 10.1042/bj1030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg D. Fatty acid mobilization--mechanisms of regulation and metabolic consequences. Biochem Soc Symp. 1963;24:111–143. [PubMed] [Google Scholar]
- Zammit V. A., Newsholme E. A. The maximum activities of hexokinase, phosphorylase, phosphofructokinase, glycerol phosphate dehydrogenases, lactate dehydrogenase, octopine dehydrogenase, phosphoenolpyruvate carboxykinase, nucleoside diphosphatekinase, glutamate-oxaloacetate transaminase and arginine kinase in relation to carbohydrate utilization in muscles from marine invertebrates. Biochem J. 1976 Dec 15;160(3):447–462. doi: 10.1042/bj1600447. [DOI] [PMC free article] [PubMed] [Google Scholar]


