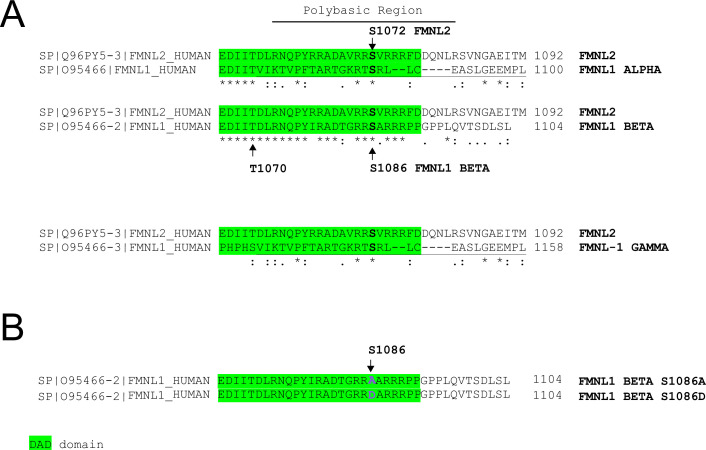
Figure 1. C-terminal alignment of FMNL1 isoforms.
S1086 of FMNL1β shows a high degree of homology with protein kinase C (PKC)-phosphorylated S1072 in FMNL2. (A) Amino acid sequences of the C-termini of FMNL2, FMNL1α, and FMNL1β, as well as FMNL1γ containing a C-terminal intron retention, and sharing the final C-terminal amino acids with FMNL1α (shown underlined). The three FMNL1 isoforms share identical sequence (100% identity) from amino acid residue 1 to T1070, and diverge in the C-terminal region, which includes the Diaphanous autoinhibitory domain (DAD) autoinhibitory domain (Han et al., 2009). The DAD domain sequence, which is responsible for autoinhibition in the murine homolog, is highlighted in green (Han et al., 2009), and the identical C-terminal amino acids shared by FMNL1α and FMNL1γ are underlined. The arginine-rich, polybasic region common to other formins such as FMNL2 (Wang et al., 2015), FHOD3 (Zhou et al., 2017), and FHOD1 (Takeya et al., 2008) (see Discussion) is also shown overlined. (B) C-terminal amino acid sequences of the two different point mutations at S1086 in FMNL1β used in this study.