Abstract
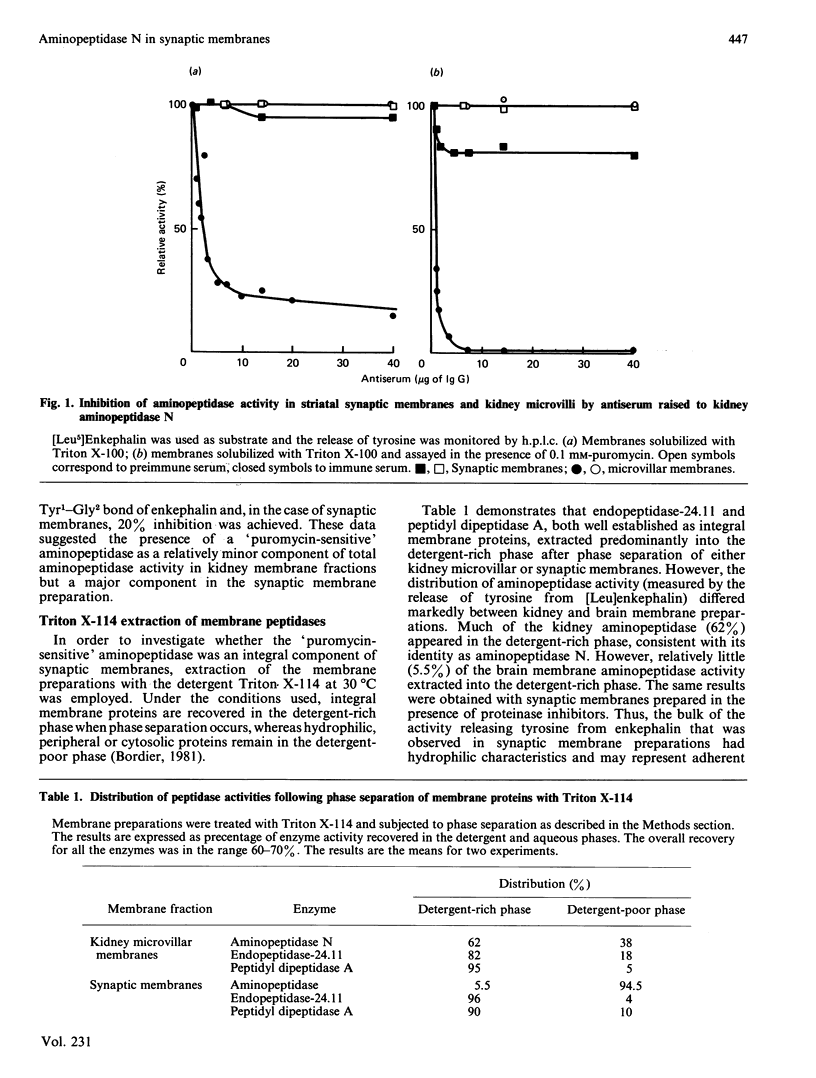
The property of solutions of Triton X-114 to separate into detergent-rich and detergent-poor phases at 30 degrees C has been exploited to investigate the identities of the aminopeptidases in synaptic membrane preparations from pig striatum. When titrated with an antiserum to aminopeptidase N (EC 3.4.11.2), synaptic membranes solubilized with Triton X-100 revealed that this enzyme apparently comprises no more than 5% of the activity releasing tyrosine from [Leu]enkephalin. When assayed in the presence of puromycin, this proportion increased to 20%. Three integral membrane proteins were fractionated by phase separation in Triton X-114. Aminopeptidase activity, endopeptidase-24.11 and peptidyl dipeptidase A partitioned predominantly into the detergent-rich phase when kidney microvillar membranes were so treated. However, only 5.5% of synaptic membrane aminopeptidase activity partitioned into this phase, although the other peptidases behaved predictably. About half of the aminopeptidase activity in the detergent-rich phase could now be titrated with the antiserum, showing that aminopeptidase N is an integral membrane protein of this preparation. Three aminopeptidase inhibitors were investigated for their ability to discriminate between the different activities revealed by these experiments. Although amastatin was the most potent (IC50 = 5 X 10(-7) M) it failed to discriminate between pure kidney aminopeptidase N, the total activity of solubilized synaptic membranes and that in the Triton X-114-rich phase. Bestatin was slightly more potent for total activity (IC50 = 6.3 X 10(-6) M) than for the other two forms (IC50 = 1.6 X 10(-5) M). Puromycin was a weak inhibitor, but was more selective. The activity of solubilized membranes was more sensitive (IC50 = 1.6 X 10(-5) M) than that of the pure enzyme or the Triton X-114-rich phase (IC50 = 4 X 10(-4) M). We suggest that the puromycin-sensitive aminopeptidase activity that predominates in crude synaptic membrane preparations may be a cytosolic contaminant or peripheral membrane protein rather than an integral membrane component. Aminopeptidase N may contribute to the extracellular metabolism of enkephalin and other susceptible neuropeptides in the brain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benuck M., Marks N. Co-identity of brain angiotensin converting enzyme with a membrane bound dipeptidyl carboxypeptidase inactivating Met - enkephalin. Biochem Biophys Res Commun. 1979 May 14;88(1):215–221. doi: 10.1016/0006-291x(79)91718-2. [DOI] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Craves F. B., Law P. Y., Hunt C. A., Loh H. H. The metabolic disposition of radiolabeled enkephalins in vitro and in situ. J Pharmacol Exp Ther. 1978 Aug;206(2):492–506. [PubMed] [Google Scholar]
- Fulcher I. S., Kenny A. J. Proteins of the kidney microvillar membrane. The amphipathic forms of endopeptidase purified from pig kidneys. Biochem J. 1983 Jun 1;211(3):743–753. doi: 10.1042/bj2110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S. G., Kenny J. Studies on the enzymology of purified preparations of brush border from rabbit kidney. Biochem J. 1973 May;134(1):43–57. doi: 10.1042/bj1340043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenstein C., Snyder S. H. Two distinct enkephalinases: solubilization, partial purification and separation from angiotensin converting enzyme. Life Sci. 1979 Dec 10;25(24-25):2065–2070. doi: 10.1016/0024-3205(79)90198-x. [DOI] [PubMed] [Google Scholar]
- Hambrook J. M., Morgan B. A., Rance M. J., Smith C. F. Mode of deactivation of the enkephalins by rat and human plasma and rat brain homogenates. Nature. 1976 Aug 26;262(5571):782–783. doi: 10.1038/262782a0. [DOI] [PubMed] [Google Scholar]
- Hersh L. B. Solubilization and characterization of two rat brain membrane-bound aminopeptidases active on Met-enkephalin. Biochemistry. 1981 Apr 14;20(8):2345–2350. doi: 10.1021/bi00511a042. [DOI] [PubMed] [Google Scholar]
- Hudgin R. L., Charleson S. E., Zimmerman M., Mumford R., Wood P. L. Enkephalinase: selective peptide inhibitors. Life Sci. 1981 Dec 21;29(25):2593–2601. doi: 10.1016/0024-3205(81)90632-9. [DOI] [PubMed] [Google Scholar]
- Hui K. S., Wang Y. J., Lajtha A. Purification and characterization of an enkephalin aminopeptidase from rat brain membranes. Biochemistry. 1983 Mar 1;22(5):1062–1067. doi: 10.1021/bi00274a010. [DOI] [PubMed] [Google Scholar]
- Kenny A. J., Maroux S. Topology of microvillar membrance hydrolases of kidney and intestine. Physiol Rev. 1982 Jan;62(1):91–128. doi: 10.1152/physrev.1982.62.1.91. [DOI] [PubMed] [Google Scholar]
- Knight M., Klee W. A. The relationship between enkephalin degradation and opiate receptor occupancy. J Biol Chem. 1978 Jun 10;253(11):3843–3847. [PubMed] [Google Scholar]
- Lane A. C., Rance M. J., Walter D. S. Subcellular localisation of leucine-enkephalin-hydrolysing activity in rat brain. Nature. 1977 Sep 1;269(5623):75–76. doi: 10.1038/269075a0. [DOI] [PubMed] [Google Scholar]
- Malfroy B., Swerts J. P., Guyon A., Roques B. P., Schwartz J. C. High-affinity enkephalin-degrading peptidase in brain is increased after morphine. Nature. 1978 Nov 30;276(5687):523–526. doi: 10.1038/276523a0. [DOI] [PubMed] [Google Scholar]
- Matsas R., Fulcher I. S., Kenny A. J., Turner A. J. Substance P and [Leu]enkephalin are hydrolyzed by an enzyme in pig caudate synaptic membranes that is identical with the endopeptidase of kidney microvilli. Proc Natl Acad Sci U S A. 1983 May;80(10):3111–3115. doi: 10.1073/pnas.80.10.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R., Kenny A. J., Turner A. J. The metabolism of neuropeptides. The hydrolysis of peptides, including enkephalins, tachykinins and their analogues, by endopeptidase-24.11. Biochem J. 1984 Oct 15;223(2):433–440. doi: 10.1042/bj2230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R., Rattray M., Kenny A. J., Turner A. J. The metabolism of neuropeptides. Endopeptidase-24.11 in human synaptic membrane preparations hydrolyses substance P. Biochem J. 1985 Jun 1;228(2):487–492. doi: 10.1042/bj2280487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R., Turner A. J., Kenny A. J. Endopeptidase-24.11 and aminopeptidase activity in brain synaptic membranes are jointly responsible for the hydrolysis of cholecystokinin octapeptide (CCK-8). FEBS Lett. 1984 Sep 17;175(1):124–128. doi: 10.1016/0014-5793(84)80583-9. [DOI] [PubMed] [Google Scholar]
- Meek J. L., Yang H. Y., Costa E. Enkephalin catabolism in vitro and in vivo. Neuropharmacology. 1977 Feb;16(2):151–154. doi: 10.1016/0028-3908(77)90064-8. [DOI] [PubMed] [Google Scholar]
- Relton J. M., Gee N. S., Matsas R., Turner A. J., Kenny A. J. Purification of endopeptidase-24.11 ('enkephalinase') from pig brain by immunoadsorbent chromatography. Biochem J. 1983 Dec 1;215(3):519–523. doi: 10.1042/bj2150519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerts J. P., Perdrisot R., Malfroy B., Schwartz J. C. Is 'enkephalinase' identical with 'angiotensin-converting enzymes'? Eur J Pharmacol. 1979 Jan 1;53(2):209–210. doi: 10.1016/0014-2999(79)90167-5. [DOI] [PubMed] [Google Scholar]
- Vogel Z., Altstein M. The effect of puromycin on the biological activity of Leu-enkephalin. FEBS Lett. 1979 Feb 1;98(1):44–48. doi: 10.1016/0014-5793(79)80148-9. [DOI] [PubMed] [Google Scholar]


