Abstract
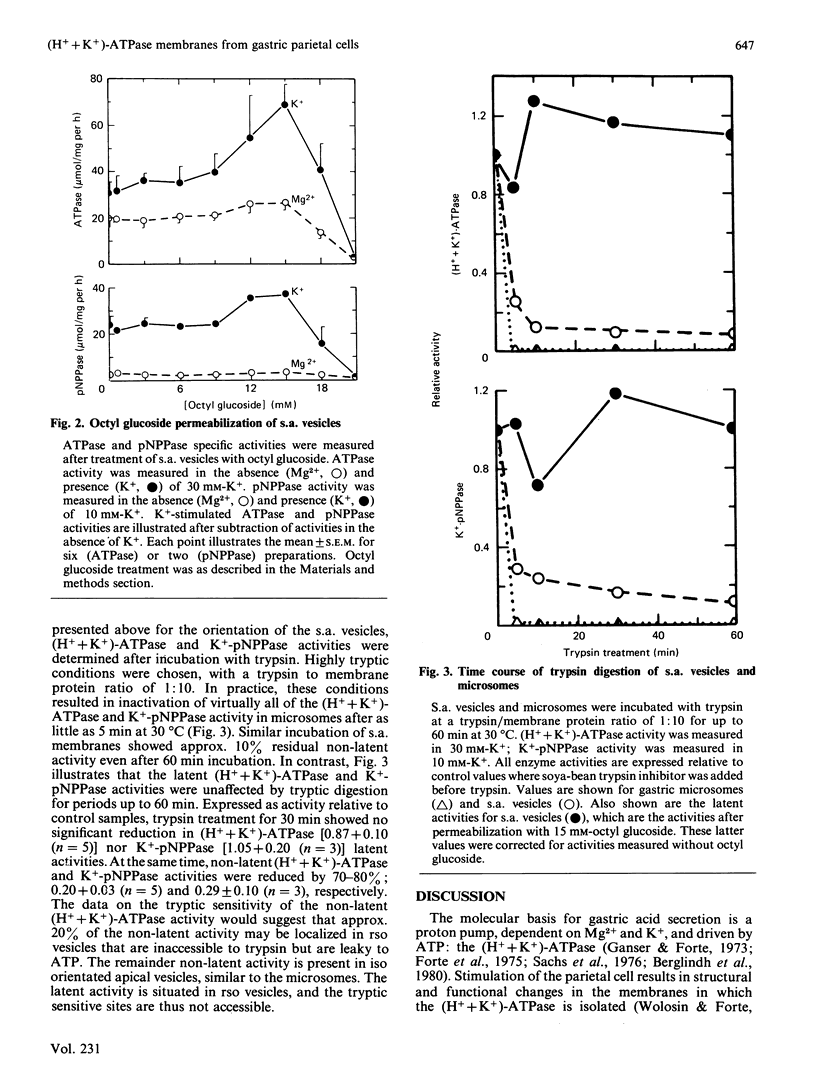
When isolated from resting parietal cells, the majority of the (H+ + K+)-ATPase activity was recovered in the microsomal fraction. These microsomal vesicles demonstrated a low K+ permeability, such that the addition of valinomycin resulted in marked stimulation of (H+ + K+)-ATPase activity, and proton accumulation. When isolated from stimulated parietal cells, the (H+ + K+)-ATPase was redistributed to larger, denser vesicles: stimulation-associated (s.a.) vesicles. S.a. vesicles showed an increased K+ permeability, such that maximal (H+ + K+)-ATPase and proton accumulation activities were observed in low K+ concentrations and no enhancement of activities occurred on the addition of valinomycin. The change in subcellular distribution of (H+ + K+)-ATPase correlated with morphological changes observed with stimulation of parietal cells, the microsomes and s.a. vesicles derived from the intracellular tubulovesicles and the apical plasma membrane, respectively. Total (H+ + K+)-ATPase activity recoverable from stimulated gastric mucosa was 64% of that from resting tissue. Therefore, we tested for latent activity in s.a. vesicles. Permeabilization of s.a. vesicles with octyl glucoside increased (H+ + K+)-ATPase activity by greater than 2-fold. Latent (H+ + K+)-ATPase activity was resistant to highly tryptic conditions (which inactivated all activity in gastric microsomes). About 20% of the non-latent (H+ + K+)-ATPase activity was also resistant to trypsin digestion. We interpret these results as indicating that, of the s.a. vesicles, approx. 55% have a right-side-out orientation and are impermeable to ATP, 10% right-side-out and permeable to ATP, and 35% have an inside-out orientation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beesley R. C., Forte J. G. Glycoproteins and glycolipids of oxyntic cell microsomes. I. Glycoproteins: carbohydrate composition, analytical and preparative fractionation. Biochim Biophys Acta. 1973 May 11;307(2):372–385. doi: 10.1016/0005-2736(73)90103-x. [DOI] [PubMed] [Google Scholar]
- Berglindh T., Dibona D. R., Pace C. S., Sachs G. ATP dependence of H+ secretion. J Cell Biol. 1980 May;85(2):392–401. doi: 10.1083/jcb.85.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Culp D. J., Forte J. G. An enriched preparation of basal-lateral plasma membranes from gastric glandular cells. J Membr Biol. 1981 Apr 15;59(2):135–142. doi: 10.1007/BF01875711. [DOI] [PubMed] [Google Scholar]
- Forte J. G., Black J. A., Forte T. M., Machen T. E., Wolosin J. M. Ultrastructural changes related to functional activity in gastric oxyntic cells. Am J Physiol. 1981 Nov;241(5):G349–G358. doi: 10.1152/ajpgi.1981.241.5.G349. [DOI] [PubMed] [Google Scholar]
- Forte J. G., Ganser A., Beesley R., Forte T. M. Unique enzymes of purified microsomes from pig fundic mucosa. K+-stimulated adenosine triphosphatase and K+-stimulated pNPPase. Gastroenterology. 1975 Jul;69(1):175–189. [PubMed] [Google Scholar]
- Forte J. G., Lee H. C. Gastric adenosine triphosphatases: a review of their possible role in HCl secretion. Gastroenterology. 1977 Oct;73(4 Pt 2):921–926. [PubMed] [Google Scholar]
- Forte T. M., Machen T. E., Forte J. G. Ultrastructural changes in oxyntic cells associated with secretory function: a membrane-recycling hypothesis. Gastroenterology. 1977 Oct;73(4 Pt 2):941–955. [PubMed] [Google Scholar]
- Ganser A. L., Forte J. G. K + -stimulated ATPase in purified microsomes of bullfrog oxyntic cells. Biochim Biophys Acta. 1973 Apr 25;307(1):169–180. doi: 10.1016/0005-2736(73)90035-7. [DOI] [PubMed] [Google Scholar]
- Im W. B., Blakeman D. P., Fieldhouse J. M., Rabon E. C. Effect of carbachol or histamine stimulation on rat gastric membranes enriched in (H+-K+)-ATPase. Biochim Biophys Acta. 1984 May 16;772(2):167–175. doi: 10.1016/0005-2736(84)90040-3. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Breitbart H., Berman M., Forte J. G. Potassium-stimulated ATPase activity and hydrogen transport in gastric microsomal vesicles. Biochim Biophys Acta. 1979 May 3;553(1):107–131. doi: 10.1016/0005-2736(79)90034-8. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Forte J. G. A study of H+ transport in gastric microsomal vesicles using fluorescent probes. Biochim Biophys Acta. 1978 Apr 4;508(2):339–356. doi: 10.1016/0005-2736(78)90336-x. [DOI] [PubMed] [Google Scholar]
- Lee J., Simpson G., Scholes P. An ATPase from dog gastric mucosa: changes of outer pH in suspensions of membrane vesicles accompanying ATP hydrolysis. Biochem Biophys Res Commun. 1974 Sep 23;60(2):825–832. doi: 10.1016/0006-291x(74)90315-5. [DOI] [PubMed] [Google Scholar]
- Munro H. N. The determination of nucleic acids. Methods Biochem Anal. 1966;14:113–176. doi: 10.1002/9780470110324.ch5. [DOI] [PubMed] [Google Scholar]
- SMITH L. Spectrophotometric assay of cytochrome c oxidase. Methods Biochem Anal. 1955;2:427–434. doi: 10.1002/9780470110188.ch13. [DOI] [PubMed] [Google Scholar]
- Saccomani G., Stewart H. B., Shaw D., Lewin M., Sachs G. Characterization of gastric mucosal membranes. IX. Fractionation and purification of K+-ATPase-containing vesicles by zonal centrifugation and free-flow electrophoresis technique. Biochim Biophys Acta. 1977 Mar 1;465(2):311–330. doi: 10.1016/0005-2736(77)90081-5. [DOI] [PubMed] [Google Scholar]
- Sachs G., Chang H. H., Rabon E., Schackman R., Lewin M., Saccomani G. A nonelectrogenic H+ pump in plasma membranes of hog stomach. J Biol Chem. 1976 Dec 10;251(23):7690–7698. [PubMed] [Google Scholar]
- Sanui H. Measurement of inorganic orthophosphate in biological materials: extraction properties of butyl acetate. Anal Biochem. 1974 Aug;60(2):489–504. doi: 10.1016/0003-2697(74)90259-0. [DOI] [PubMed] [Google Scholar]
- Smolka A., Helander H. F., Sachs G. Monoclonal antibodies against gastric H+ + K+ ATPase. Am J Physiol. 1983 Oct;245(4):G589–G596. doi: 10.1152/ajpgi.1983.245.4.G589. [DOI] [PubMed] [Google Scholar]
- Wolosin J. M., Forte J. G. Changes in the membrane environment of the (K+ + H+)-ATPase following stimulation of the gastric oxyntic cell. J Biol Chem. 1981 Apr 10;256(7):3149–3152. [PubMed] [Google Scholar]
- Wolosin J. M., Forte J. G. Functional differences between K+-ATPase rich membranes isolated from resting or stimulated rabbit fundic mucosa. FEBS Lett. 1981 Mar 23;125(2):208–212. doi: 10.1016/0014-5793(81)80720-x. [DOI] [PubMed] [Google Scholar]
- Wolosin J. M., Forte J. G. Stimulation of oxyntic cell triggers K+ and Cl- conductances in apical H+-K+-ATPase membrane. Am J Physiol. 1984 May;246(5 Pt 1):C537–C545. doi: 10.1152/ajpcell.1984.246.5.C537. [DOI] [PubMed] [Google Scholar]
- Wolosin J. M., Okamoto C., Forte T. M., Forte J. G. Actin and associated proteins in gastric epithelial cells. Biochim Biophys Acta. 1983 Dec 13;761(2):171–182. doi: 10.1016/0304-4165(83)90226-x. [DOI] [PubMed] [Google Scholar]


