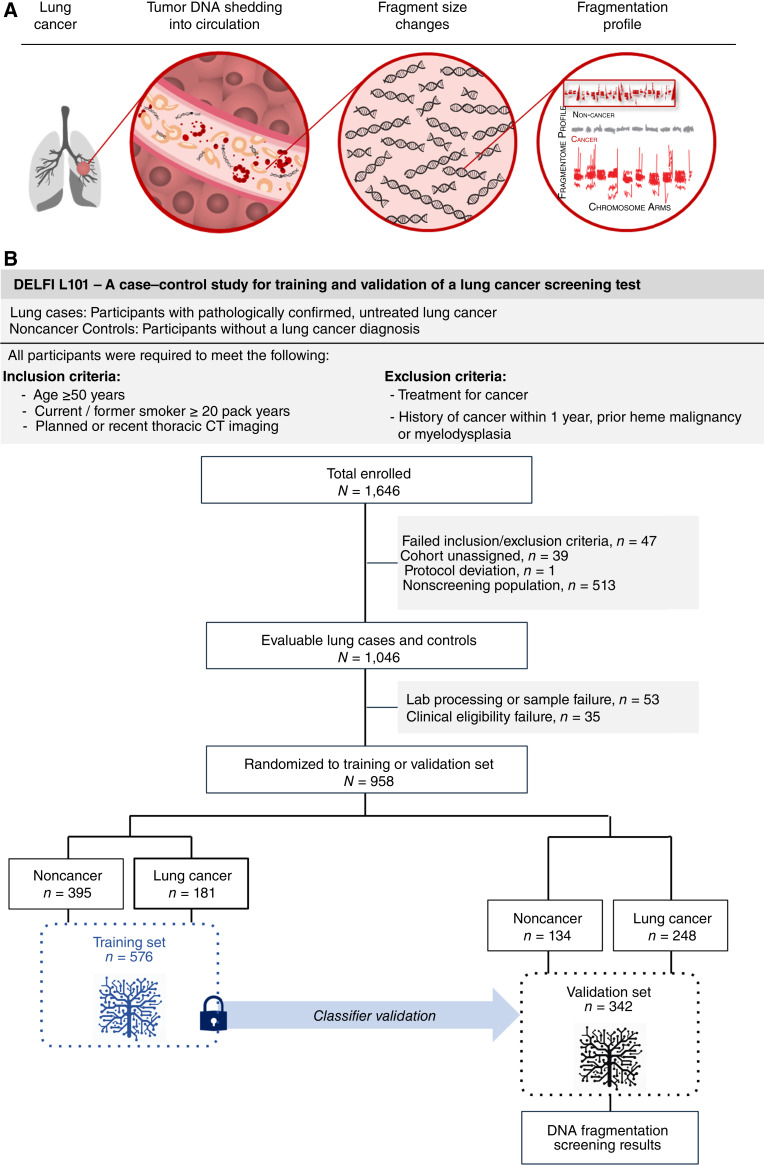
Figure 1.
Overall approach to clinical validation of a cell-free DNA fragmentome assay for augmentation of lung cancer early detection. A, Illustration representing the DELFI approach for lung cancer through noninvasive assessment of cell-free DNA fragmentation profiles (ratio of short to long cfDNA fragments). Nucleosomal DNA with variable length of linker DNA is released by dying lung cancer cells into the circulation. Genome-wide mapping of the cfDNA fragments demonstrates more aberrant profiles with cancer cell cfDNA fragments compared with the cfDNA in noncancer individuals. B, The DNA Evaluation of Fragments for Early Interception–Lung Cancer Training Study, DELFI-L101 study was a prospective case–control study (NCT04825834, including two institutional supplementary protocols NCT00301119 and NCT01775072). The flow diagram illustrates the inclusion and exclusion of L101 participants based on clinical, sample, and assay eligibility criteria and the assignment of evaluable participants to the classifier training (n = 576) and clinical validation (n = 382) sets. Machine learning of genome-wide cfDNA fragmentation profiles from the training set was used to develop a locked classifier that was evaluated in the clinical validation set.