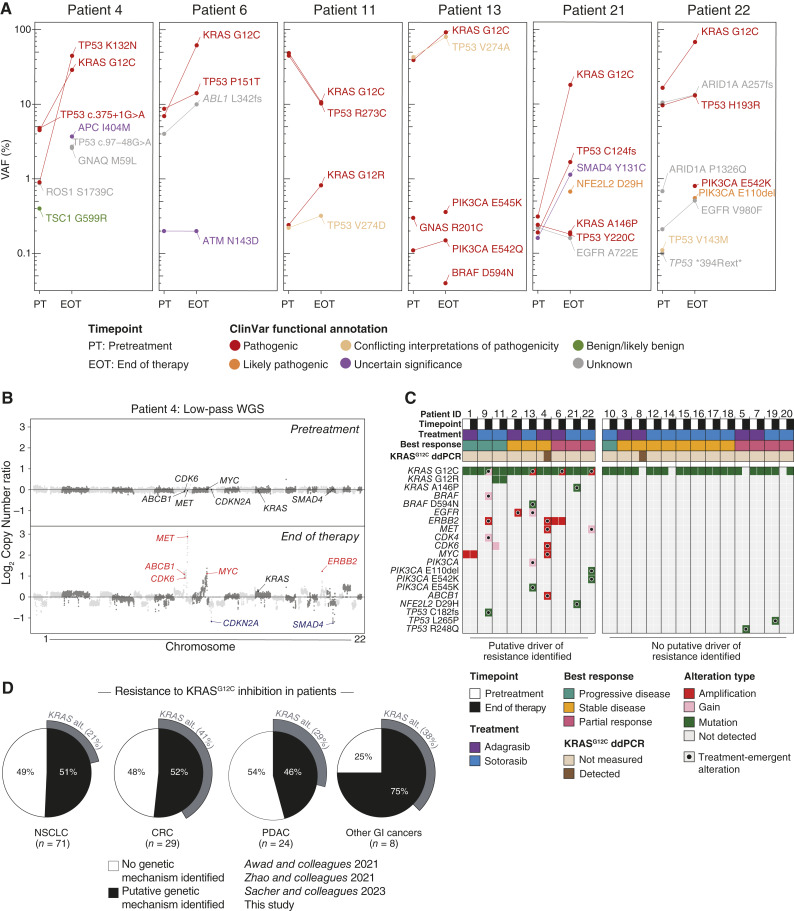
Figure 1.
Genetic alterations associated with acquired resistance to KRASG12C inhibition in patients with PDAC. A, Variant allele frequencies (VAF) for the indicated genomic variants in pretreatment (PT) and end of therapy (EOT) ctDNA samples across patients with PDAC treated with adagrasib monotherapy on the KRYSTAL-1 trial (patients 4, 6) or sotorasib monotherapy on the CodeBreaK100 trial (patients 11, 13, 21, 22). B, Paired genome-wide copy number profiles from low-pass whole genome sequencing (organized by chromosome and genomic coordinates) from PT and EOT ctDNA samples for patient 4 treated with adagrasib. Gene labels indicate amplified putative drivers of acquired resistance or tumor suppressor genes. C, Co-mutation plot displaying pathogenic and likely pathogenic treatment-emergent variants and genomic amplification of putative drivers of resistance detected in ctDNA samples from PDAC patients with acquired resistance to adagrasib (patients 1–8) or sotorasib (patients 9–22). The best radiographic treatment response is indicated as measured by RECIST criteria. D, Aggregated classification of mechanisms of resistance to KRASG12C inhibition across cancer types. Data include patients from this study and previously published studies (Supplementary Table S2; Supplementary Fig. S1G).