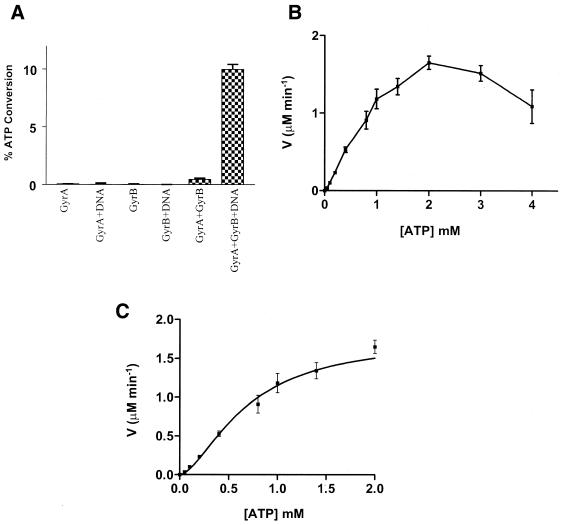
Figure 3.
ATPase activity of M.smegmatis DNA gyrase. (A) Intrinsic and DNA-stimulated ATPase activity of purified individual subunits and holoenzyme. Assays were carried out with 450 nM each of GyrA and GyrB, in the presence of 1.4 mM ATP and 10 µg ml–1 linear DNA. (B) The rate of DNA-dependent ATP hydrolysis by mycobacterial DNA gyrase (75 nM) at various substrate concentrations ranging from 0.05 to 4 mM ATP, in the presence of 10 µg ml–1 240 bp DNA fragment. (C) Non-linear regression analysis of ATP hydrolysis rates with ATP concentrations of 0.05–2.0 mM. All the experiments were repeated three times independently and plotted.