Abstract
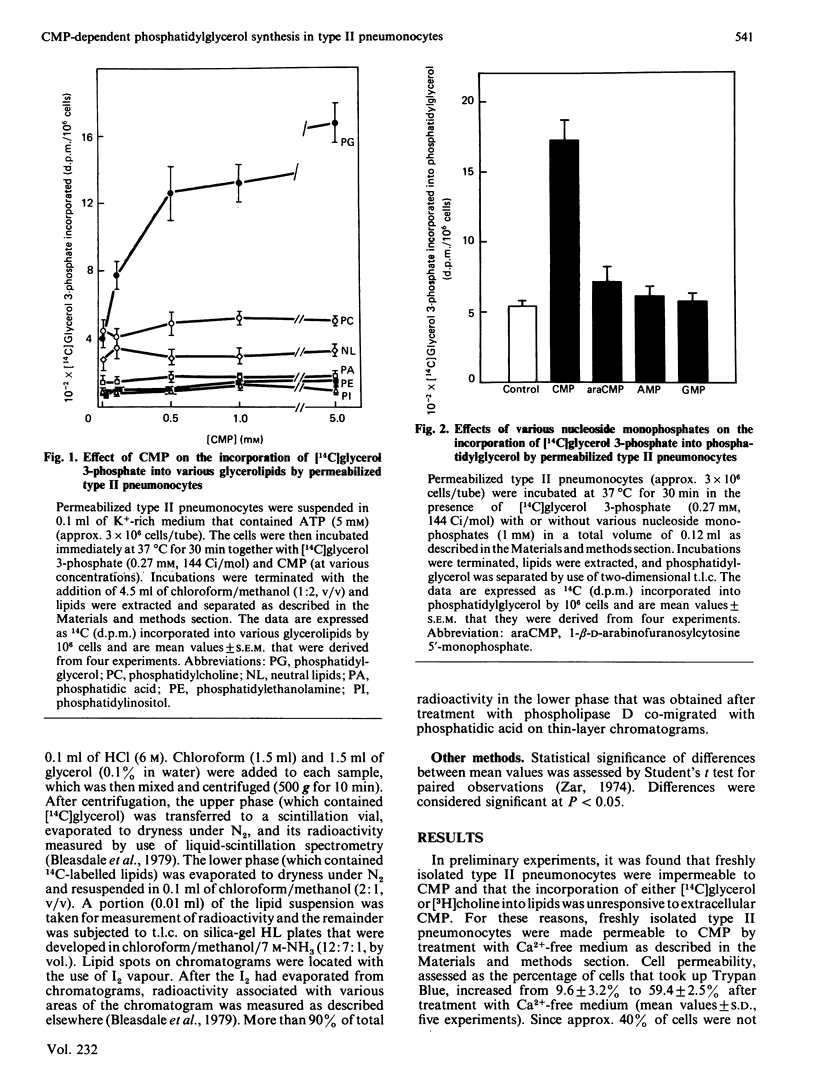
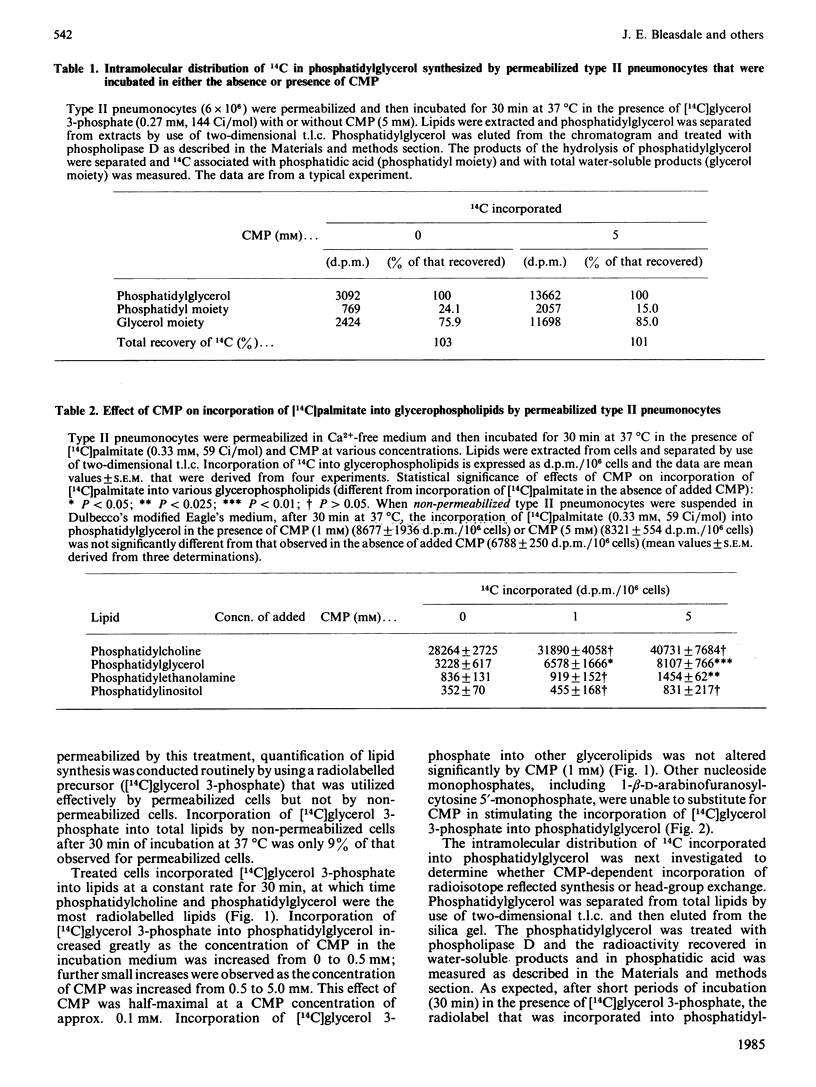
Results of previous investigations support the proposition that, in type II pneumonocytes, CMP is involved in integration of the synthesis of phosphatidylcholine and phosphatidylglycerol for lung surfactant. In the present investigation, the amount of CMP in rat type II pneumonocytes was altered directly and resultant changes in the synthesis of phosphatidylglycerol were examined. Type II pneumonocytes were made permeable to CMP by treatment with Ca2+-free medium, and phosphatidylglycerol synthesis was then assessed by measurement of the incorporation of a radiolabelled precursor, [14C]glycerol 3-phosphate, that was not effectively utilized by cells that resisted permeabilization. Incorporation of [14C]glycerol 3-phosphate into phosphatidylglycerol (but not into other lipids) was stimulated greatly by CMP (half-maximal stimulation at approx. 0.1 mM). CMP stimulated the incorporation of [14C]glycerol 3-phosphate into both the phosphatidyl moiety and the head group of phosphatidylglycerol. Incorporation of [14C]palmitate into phosphatidylglycerol was also stimulated by CMP. myo-Inositol, at concentrations found in foetal-rat serum (0.2-2.0 mM), inhibited CMP-dependent incorporation of [14C]glycerol 3-phosphate into phosphatidylglycerol and promoted, instead, CMP-dependent incorporation into phosphatidylinositol. These data, when extrapolated to foetal type II pneumonocytes, are consistent with the view that the developmental increase in the synthesis of phosphatidylglycerol for surfactant by foetal lungs is promoted by the increase in intracellular CMP and the declining availability of myo-inositol that were found previously to be associated with this period of development.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akesson B., Sundler R. Factors controlling the biosynthesis of individual phosphoglycerides in liver. Biochem Soc Trans. 1977;5(1):43–48. doi: 10.1042/bst0050043. [DOI] [PubMed] [Google Scholar]
- Anceschi M. M., Di Renzo G. C., Venincasa M. D., Bleasdale J. E. The choline-depleted type II pneumonocyte. A model for investigating the synthesis of surfactant lipids. Biochem J. 1984 Nov 15;224(1):253–262. doi: 10.1042/bj2240253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batenburg J. J., Klazinga W., van Golde L. M. Regulation of phosphatidylglycerol and phosphatidylinositol synthesis in alveolar type II cells isolated from adult rat lung. FEBS Lett. 1982 Oct 18;147(2):171–174. doi: 10.1016/0014-5793(82)81035-1. [DOI] [PubMed] [Google Scholar]
- Bleasdale J. E., Johnston J. M. CMP-dependent incorporation of [14C]Glycerol 3-phosphate into phosphatidylglycerol and phosphatidylglycerol phosphate by rabbit lung microsomes. Biochim Biophys Acta. 1982 Mar 12;710(3):377–390. doi: 10.1016/0005-2760(82)90121-7. [DOI] [PubMed] [Google Scholar]
- Bleasdale J. E., Maberry M. C., Quirk J. G. Myo-inositol homeostasis in foetal rabbit lung. Biochem J. 1982 Jul 15;206(1):43–52. doi: 10.1042/bj2060043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleasdale J. E., Tyler N. E., Busch F. N., Quirk J. G. The influence of myo-inositol on phosphatidylglycerol synthesis by rat type II pneumonocytes. Biochem J. 1983 Jun 15;212(3):811–818. doi: 10.1042/bj2120811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleasdale J. E., Wallis P., MacDonald P. C., Johnston J. M. Characterization of the forward and reverse reactions catalyzed by CDP-diacylglycerol:inositol transferase in rabbit lung tissue. Biochim Biophys Acta. 1979 Oct 26;575(1):135–147. doi: 10.1016/0005-2760(79)90139-5. [DOI] [PubMed] [Google Scholar]
- Burton L. E., Wells W. W. Studies on the developmental pattern of the enzymes converting glucose 6-phosphate to myo-inositol in the rat. Dev Biol. 1974 Mar;37(1):35–42. doi: 10.1016/0012-1606(74)90167-5. [DOI] [PubMed] [Google Scholar]
- Carter J. R., Kennedy E. P. Enzymatic synthesis of cytidine diphosphate diglyceride. J Lipid Res. 1966 Sep;7(5):678–683. [PubMed] [Google Scholar]
- Fallon H. J., Lamb R. G. Acylation of sn-glycerol 3-phosphate by cell fractions of rat liver. J Lipid Res. 1968 Sep;9(5):652–660. [PubMed] [Google Scholar]
- Hallman M., Gluck L. Formation of acidic phospholipids in rabbit lung during perinatal development. Pediatr Res. 1980 Nov;14(11):1250–1259. doi: 10.1203/00006450-198011000-00020. [DOI] [PubMed] [Google Scholar]
- Hallman M., Wermer D., Epstein B. L., Gluck L. Effects of maternal insulin or glucose infusion on the fetus: study on lung surfactant phospholipids, plasma myoinositol, and fetal growth in the rabbit. Am J Obstet Gynecol. 1982 Apr 1;142(7):877–882. doi: 10.1016/s0002-9378(16)32535-2. [DOI] [PubMed] [Google Scholar]
- Hirabayashi T., Larson T. J., Dowhan W. Membrane-associated phosphatidylglycerophosphate synthetase from Escherichia coli: purification by substrate affinity chromatography on cytidine 5'-diphospho-1,2-diacyl-sn-glycerol sepharose. Biochemistry. 1976 Nov 30;15(24):5205–5211. doi: 10.1021/bi00669a002. [DOI] [PubMed] [Google Scholar]
- Kikkawa Y., Yoneda K. The type II epithelial cell of the lung. I. Method of isolation. Lab Invest. 1974 Jan;30(1):76–84. [PubMed] [Google Scholar]
- Longmuir K. J., Johnston J. M. Changes in CTP:phosphatidate cytidylyltransferase activity during rabbit lung development. Biochim Biophys Acta. 1980 Dec 5;620(3):500–508. doi: 10.1016/0005-2760(80)90142-3. [DOI] [PubMed] [Google Scholar]
- Mandel P., Edel-Harth S. Free nucleotides in the rat brain during post-natal development. J Neurochem. 1966 Jul;13(7):591–595. doi: 10.1111/j.1471-4159.1966.tb11955.x. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Williams M. C., Greenleaf R. D., Clements J. A. Isolation and properties of type II alveolar cells from rat lung. Am Rev Respir Dis. 1977 Jun;115(6):1015–1026. doi: 10.1164/arrd.1977.115.6.1015. [DOI] [PubMed] [Google Scholar]
- Possmayer F. CDP-choline reversal of the CMP and CTP inhibition of phosphatidic acid synthesis by rat brain preparations. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1415–1426. doi: 10.1016/s0006-291x(74)80441-9. [DOI] [PubMed] [Google Scholar]
- Possmayer F., Meiners B., Mudd J. B. Regulation by cytidine nucleotides of the acylation of sn-(14C)glycerol 3-phosphate. Regional and subcellular distribution of the enzymes responsible for phosphatidic acid synthesis de novo in the central nervous system of the rat. Biochem J. 1973 Mar;132(3):381–394. doi: 10.1042/bj1320381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post M., Batenburg J. J., Smith B. T., Van Golde L. M. Pool sizes of precursors for phosphatidylcholine formation in adult rat lung type II cells. Biochim Biophys Acta. 1984 Oct 4;795(3):552–557. doi: 10.1016/0005-2760(84)90185-1. [DOI] [PubMed] [Google Scholar]
- Quirk J. G., Bleasdale J. E., MacDonald P. C., Johnston J. M. A role for cytidine monophosphate in the regulation of the glycerophospholipid composition of surfactant in developing lung. Biochem Biophys Res Commun. 1980 Aug 14;95(3):985–992. doi: 10.1016/0006-291x(80)91570-3. [DOI] [PubMed] [Google Scholar]
- Quirk J. G., Jr, Baumgarten B., Bleasdale J. E. Effect of myo-inositol on the glycerophospholipid composition of adult and fetal rat lung tissue. J Perinat Med. 1984;12(4):201–210. doi: 10.1515/jpme.1984.12.4.201. [DOI] [PubMed] [Google Scholar]
- Raetz C. R., Chu M. Y., Srivastava S., Turcotte J. G. A phospholipid derivative of cytosine arabinoside and its conversion to phosphatidylinositol by animal tissue. Science. 1977 Apr 15;196(4287):303–305. doi: 10.1126/science.191910. [DOI] [PubMed] [Google Scholar]
- Streb H., Schulz I. Regulation of cytosolic free Ca2+ concentration in acinar cells of rat pancreas. Am J Physiol. 1983 Sep;245(3):G347–G357. doi: 10.1152/ajpgi.1983.245.3.G347. [DOI] [PubMed] [Google Scholar]
- Yavin E., Zutra A. Separation and analysis of 32P-labeled phospholipids by a simple and rapid thin-layer chromatographic procedure and its application to cultured neuroblastoma cells. Anal Biochem. 1977 Jun;80(2):430–437. doi: 10.1016/0003-2697(77)90665-0. [DOI] [PubMed] [Google Scholar]
- Zborowski J., Wojtczak L. Phospholipid synthesis in rat liver mitochondria. Biochim Biophys Acta. 1969 Jul 29;187(1):73–84. doi: 10.1016/0005-2760(69)90134-9. [DOI] [PubMed] [Google Scholar]


