Abstract
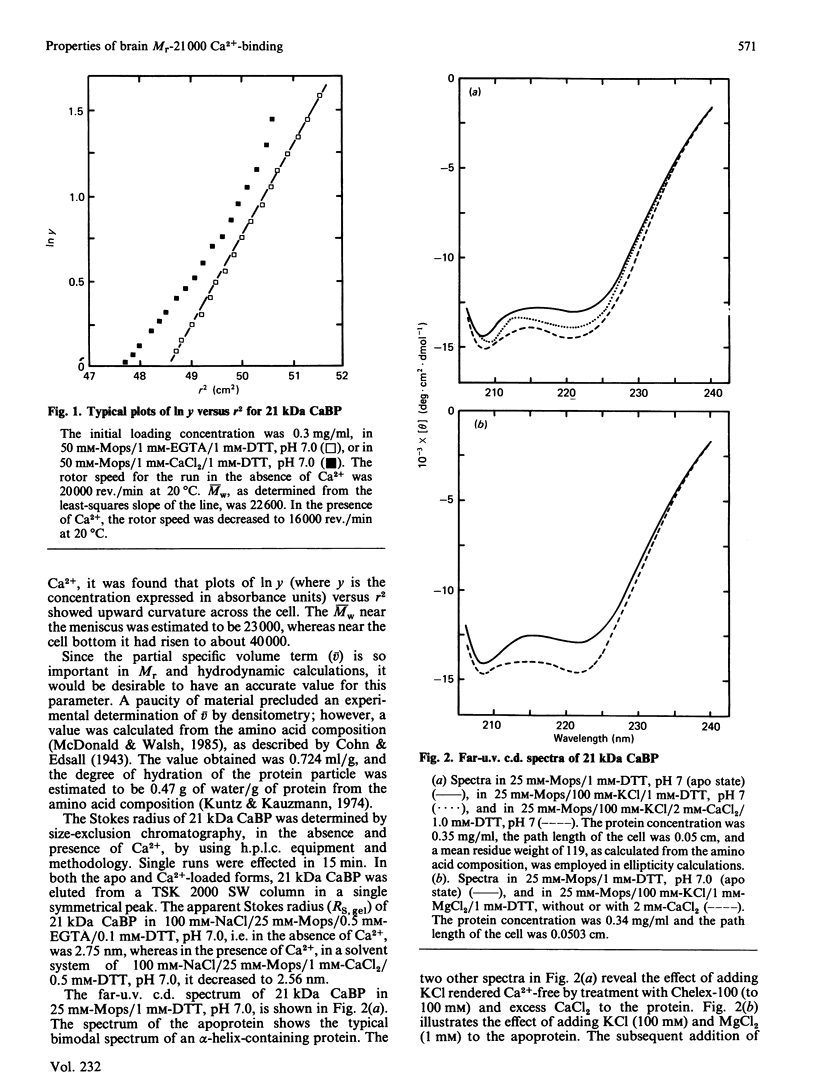
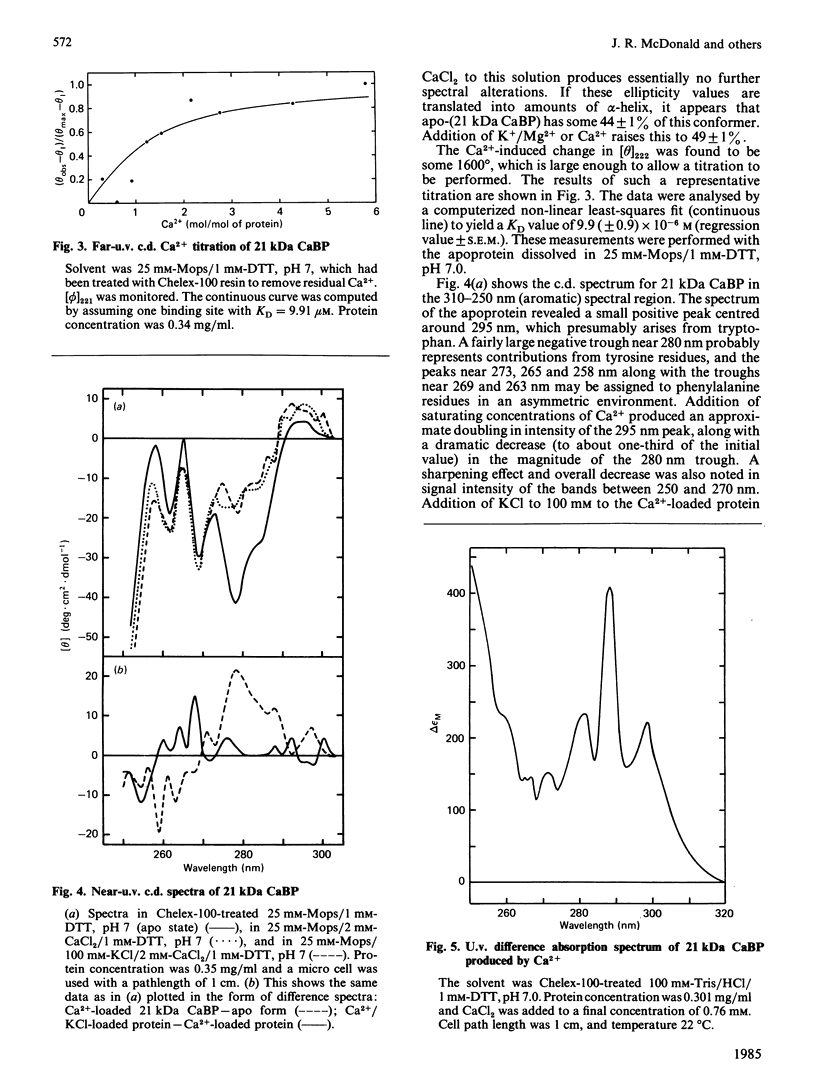
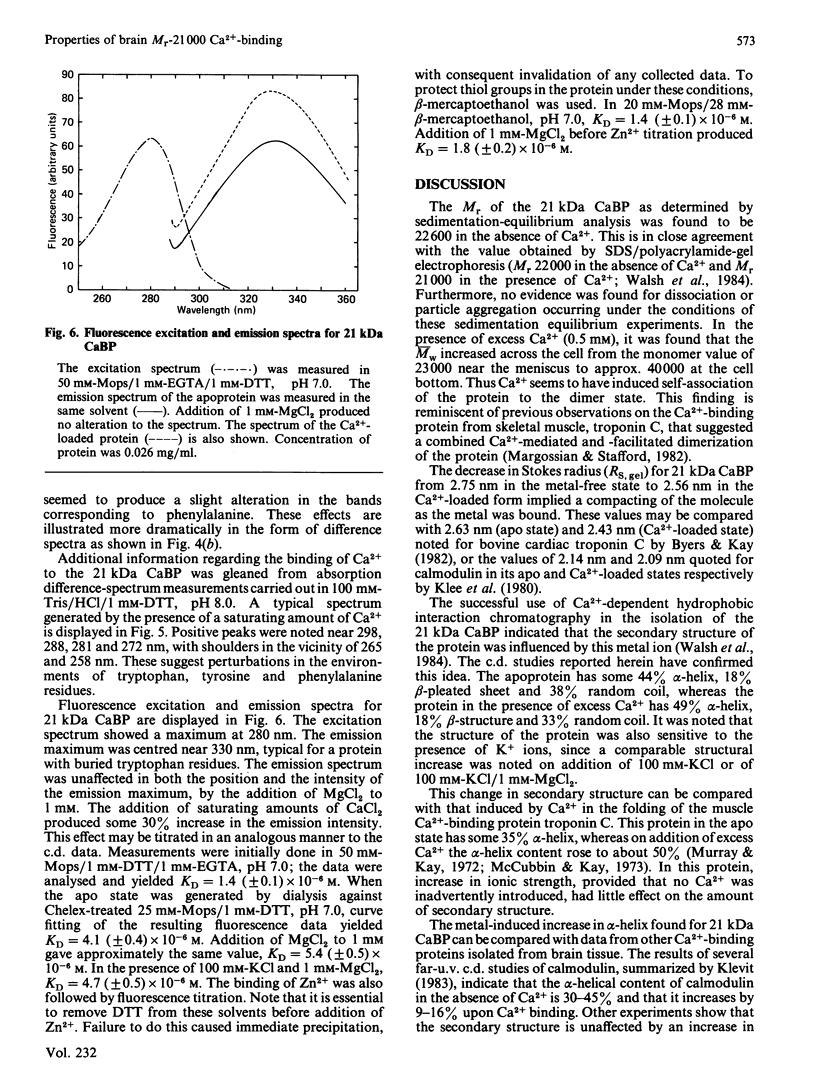
The physicochemical properties of a novel Mr-21 000 Ca2+-binding protein isolated from bovine brain were investigated. The protein exhibited a partial specific volume of 0.724 ml/g, a degree of hydration of 0.47 g of water/g of protein and a mean residue weight of 119. Sedimentation equilibrium analysis revealed Mr = 22 600 in the absence of Ca2+; Ca2+ binding appeared to induce dimerization of the molecule. Size-exclusion chromatography indicated a compacting of the molecule on binding of Ca2+: the Stokes radius decreased from 2.75 nm in the absence of Ca2+ to 2.56 nm in its presence. Far-u.v.c.d. spectroscopy showed the apoprotein to be composed of 44% alpha-helix, 18% beta-pleated sheet and 38% random coil. Addition of either KCl (0.1 M) plus Mg2+ (1 mM), or Ca2+ (2 mM), changed the conformation to 49% alpha-helix, 18% beta-pleated sheet and 33% random coil. Near-u.v.c.d. and u.v. difference spectroscopy both indicated perturbations in the environments of all three types of aromatic amino acids on binding of Ca2+. Ca2+ binding also resulted in a 30% enhancement in the tryptophan fluorescence emission intensity. Ca2+ titration of the far-u.v.c.d. and fluorescence enhancement provided KD values of 9.91 microM and 4.68 microM respectively. Finally, the protein was shown to bind Zn2+ with KD = 1.44 microM (no Mg2+) and 1.82 microM (+ Mg2+). These observations strongly support the possibility that this novel Ca2+-binding protein resembles calmodulin and related Ca2+-binding proteins and undergoes a conformational change on binding of Ca2+ which reflects a physiological role in Ca2+-mediated regulation of brain function.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byers D. M., Kay C. M. Hydrodynamic properties of bovine cardiac troponin C. Biochemistry. 1982 Jan 19;21(2):229–233. doi: 10.1021/bi00531a005. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Cox J. A., Ferraz C., Demaille J. G., Perez R. O., van Tuinen D., Marmé D. Calmodulin from neurospora crassa. General properties and conformational changes. J Biol Chem. 1982 Sep 25;257(18):10694–10700. [PubMed] [Google Scholar]
- Crouch T. H., Klee C. B. Positive cooperative binding of calcium to bovine brain calmodulin. Biochemistry. 1980 Aug 5;19(16):3692–3698. doi: 10.1021/bi00557a009. [DOI] [PubMed] [Google Scholar]
- Dabrowska R., Sherry J. M., Aromatorio D. K., Hartshorne D. J. Modulator protein as a component of the myosin light chain kinase from chicken gizzard. Biochemistry. 1978 Jan 24;17(2):253–258. doi: 10.1021/bi00595a010. [DOI] [PubMed] [Google Scholar]
- Dedman J. R., Potter J. D., Jackson R. L., Johnson J. D., Means A. R. Physicochemical properties of rat testis Ca2+-dependent regulator protein of cyclic nucleotide phosphodiesterase. Relationship of Ca2+-binding, conformational changes, and phosphodiesterase activity. J Biol Chem. 1977 Dec 10;252(23):8415–8422. [PubMed] [Google Scholar]
- Klee C. B. Conformational transition accompanying the binding of Ca2+ to the protein activator of 3',5'-cyclic adenosine monophosphate phosphodiesterase. Biochemistry. 1977 Mar 8;16(5):1017–1024. doi: 10.1021/bi00624a033. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Richman P. G. Calmodulin. Annu Rev Biochem. 1980;49:489–515. doi: 10.1146/annurev.bi.49.070180.002421. [DOI] [PubMed] [Google Scholar]
- Klevit R. E. Spectroscopic analyses of calmodulin and its interactions. Methods Enzymol. 1983;102:82–104. doi: 10.1016/s0076-6879(83)02010-8. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Niggli V., Scrutton M. C. Thrombin and activators of protein kinase C modulate secretory responses of permeabilised human platelets induced by Ca2+. Eur J Biochem. 1984 Sep 3;143(2):437–446. doi: 10.1111/j.1432-1033.1984.tb08391.x. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Scrutton M. C. Cyclic nucleotides control a system which regulates Ca2+ sensitivity of platelet secretion. Nature. 1984 May 3;309(5963):66–68. doi: 10.1038/309066a0. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H. Structure and evolution of calcium-modulated proteins. CRC Crit Rev Biochem. 1980;8(2):119–174. doi: 10.3109/10409238009105467. [DOI] [PubMed] [Google Scholar]
- Kuntz I. D., Jr, Kauzmann W. Hydration of proteins and polypeptides. Adv Protein Chem. 1974;28:239–345. doi: 10.1016/s0065-3233(08)60232-6. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Wierman B. M., Storm D. R. Calcium-induced exposure of a hydrophobic surface on calmodulin. Biochemistry. 1980 Aug 5;19(16):3814–3819. doi: 10.1021/bi00557a025. [DOI] [PubMed] [Google Scholar]
- Levine B. A., Dalgarno D. C. The dynamics and function of calcium-binding proteins. Biochim Biophys Acta. 1983 Sep 15;726(3):187–204. doi: 10.1016/0304-4173(83)90005-8. [DOI] [PubMed] [Google Scholar]
- Mani R. S., Boyes B. E., Kay C. M. Physicochemical and optical studies on calcium- and potassium-induced conformational changes in bovine brain S-100b protein. Biochemistry. 1982 May 25;21(11):2607–2612. doi: 10.1021/bi00540a005. [DOI] [PubMed] [Google Scholar]
- Mani R. S., Kay C. M. Isolation and spectral studies on the calcium binding properties of bovine brain S-100a protein. Biochemistry. 1983 Aug 2;22(16):3902–3907. doi: 10.1021/bi00285a027. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Stafford W. F., 3rd Calcium-induced dimerization of troponin-C. J Biol Chem. 1982 Feb 10;257(3):1160–1165. [PubMed] [Google Scholar]
- McCubbin W. D., Kay C. M. Physicochemical and biological studies on the metal-induced conformational change in troponin A. Implication of carboxyl groups in the binding of calcium ion. Biochemistry. 1973 Oct 9;12(21):4228–4232. doi: 10.1021/bi00745a029. [DOI] [PubMed] [Google Scholar]
- McDonald J. R., Walsh M. P. Ca2+-binding proteins from bovine brain including a potent inhibitor of protein kinase C. Biochem J. 1985 Dec 1;232(2):559–567. doi: 10.1042/bj2320559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means A. R., Tash J. S., Chafouleas J. G. Physiological implications of the presence, distribution, and regulation of calmodulin in eukaryotic cells. Physiol Rev. 1982 Jan;62(1):1–39. doi: 10.1152/physrev.1982.62.1.1. [DOI] [PubMed] [Google Scholar]
- Murray A. C., Kay C. M. Hydrodynamic and optical properties of troponin A. Demonstration of a conformational change upon binding calcium ion. Biochemistry. 1972 Jul 4;11(14):2622–2627. doi: 10.1021/bi00764a012. [DOI] [PubMed] [Google Scholar]
- Seamon K. B. Calcium- and magnesium-dependent conformational states of calmodulin as determined by nuclear magnetic resonance. Biochemistry. 1980 Jan 8;19(1):207–215. doi: 10.1021/bi00542a031. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Hidaka H. Hydrophobic regions function in calmodulin-enzyme(s) interactions. J Biol Chem. 1980 Dec 10;255(23):11078–11080. [PubMed] [Google Scholar]
- Walsh M. P., Valentine K. A., Ngai P. K., Carruthers C. A., Hollenberg M. D. Ca2+-dependent hydrophobic-interaction chromatography. Isolation of a novel Ca2+-binding protein and protein kinase C from bovine brain. Biochem J. 1984 Nov 15;224(1):117–127. doi: 10.1042/bj2240117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M., Stevens F. C. Characterization of tryptic fragments obtained from bovine brain protein modulator of cyclic nucleotide phosphodiesterase. J Biol Chem. 1977 Nov 10;252(21):7440–7443. [PubMed] [Google Scholar]
- Walsh M., Stevens F. C., Oikawa K., Kay C. M. Circular dichroism studies of native and chemically modified Ca2+-dependent protein modulator. Can J Biochem. 1979 Mar;57(3):267–278. doi: 10.1139/o79-034. [DOI] [PubMed] [Google Scholar]
- Walsh M., Stevens F. C., Oikawa K., Kay C. M. Circular dichroism studies on Ca2+-dependent protein modulator oxidized with N-chlorosuccinimide. Biochemistry. 1978 Sep 19;17(19):3928–3930. [PubMed] [Google Scholar]
- Wolff D. J., Poirier P. G., Brostrom C. O., Brostrom M. A. Divalent cation binding properties of bovine brain Ca2+-dependent regulator protein. J Biol Chem. 1977 Jun 25;252(12):4108–4117. [PubMed] [Google Scholar]


