Abstract
Objective
The objective of this study is to investigate factors associated with outcomes after 3 months of instructed usage of hand-held digital devices (DD) in patients with acquired comitant esotropia (ACE).
Methods and analysis
This prospective multicentre observational study included patients with ACE, aged 5–35 years, who used DD within 1 year of onset and were followed up for clinical findings and instructed use of DD. The outcomes were classified into four groups: cured, improved, unchanged and worsened. After the analysis of group differences in the clinical and DD use-related factors by univariate analysis, we used ordinal logistic regression models to identify factors associated with favourable outcomes.
Results
Of 156 patients (mean age (SD): 16.4 (7.4) years), 10 (6%), 58 (37%), 67 (43%) and 21 (14%) were classified into the cured, improved, unchanged, and worsened, respectively. In the univariate analysis, consultation within 3 months of onset, small-angle strabismus at distance and good stereoacuity were associated with good outcomes. Ordinal logistic regression analysis on adjusting for age with stereoacuity or successful DD-use time halving showed that small-angle strabismus at distance (OR: 1.02, 95% CI 1.00 to 1.03, p=0.023), good stereoacuity (OR: 1.31; 95% CI 1.10 to 1.56; p=0.003) and successful halving of DD-use time (OR: 0.63; 95% CI 0.43 to 0.92; p=0.016) influenced favourable outcomes.
Conclusion
Patients with small-angle esotropia, good stereoacuity on consultation and success in halving DD-use time had a higher chance of recovery through instructional DD usage. Further studies using objectively measurable systems are needed to ensure the accuracy of DD-use time.
Keywords: Strabismus, Child Health, Esotropia, Depth Perception, Prospective Studies, Smartphone
WHAT IS ALREADY KNOWN ON THIS TOPIC
Acquired comitant esotropia (ACE) has reportedly increased with the widespread use of digital devices, but its clinical relevance has not been clarified.
WHAT THIS STUDY ADDS
Instructions on hand-held digital device (DD) usage in patients with ACE who were using DD at presentation resulted in cure of esotropia in 6% cases and improvement in 37% cases. In addition, factors associated with favourable outcomes included small-angle strabismus at distance, good stereoacuity and successful halving of the time spent using the DD.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Amid concerns about the impact of DD use on ocular diseases, the findings herein will be informative in guiding clinical management decisions and public health policies.
Introduction
Acute acquired comitant esotropia (AACE) is characterised by acute onset of esotropia without limitation of eye movement in children and adults.1 2 In recent years, an increase in the number of AACE cases, including ‘acute’ and ‘subacute’ cases, and cases reported several years after the onset of acquired comitant esotropia (ACE), has been reported, which is thought to be related to the widespread use of digital devices.3,8 Several studies have reported an association between the excessive use of digital devices and the development of acquired esotropia3 9 and that limiting their use improved esotropia.9 10 However, the definition of digital-device overuse remains ambiguous, and there is no standard for how long it should be reduced.
To confirm the relationship between hand-held digital device (DD) usage and ACE, we conducted a nationwide, multicentre, prospective study focusing on young patients.11 We observed changes in strabismus angle over a 3-month period following instructional DD usage. While some cases showed improvement in strabismus, the observed change was clinically modest, and the probability of cure was low, suggesting the presence of other parameters influencing the relationship between DD usage and ACE. To understand the characteristics of patients who are more likely to improve their ACE with instructions for DD usage and to clarify the relationship between DD-use time and outcomes, this study investigated the relationship between 3-month outcomes and various clinical parameters.
Materials and methods
Study design
This was a prospective multicentre observational study.
Study participants
Participants comprised patients aged 5–35 years whose diplopia or esotropia was noticed by themselves or their guardians within 1 year of their initial visit and were subsequently confirmed as having esotropia. Details of inclusion and exclusion criteria are described in our first study.11 Between November 2019 and December 2021, 221 patients from 55 centres were enrolled (online supplemental material 1). Interviews with patients or their guardians covered the onset, ocular symptoms and DD usage. Patients underwent a detailed ophthalmologic examination at the initial visit, complemented by a neurological examination with head MRI/CT whenever feasible. Patients with neurological abnormalities were excluded from this study. Those with a history of amblyopia or strabismus were included. Spectacles were prescribed for patients without appropriate refractive glasses, and prismatic glasses were provided on request. A common study manual was used to standardise examination procedure at all the facilities.
We provided the following instructions regarding the use of DD: limit usage to less than 60 min/day for elementary school students and younger, less than 120 min/day for junior high school students and older, keep a minimum viewing distance of 30 cm and take a 5-min break after every 30 min of use. Participants were instructed to maintain a diary of how long they used DD per day (DD time) and visit the hospital in the first and third months for interviews and ophthalmological examinations. Patients were instructed to record the time of using DD with honesty and accuracy; in case the patient was of primary school age or younger, they were instructed to take help of their parent or guardian.
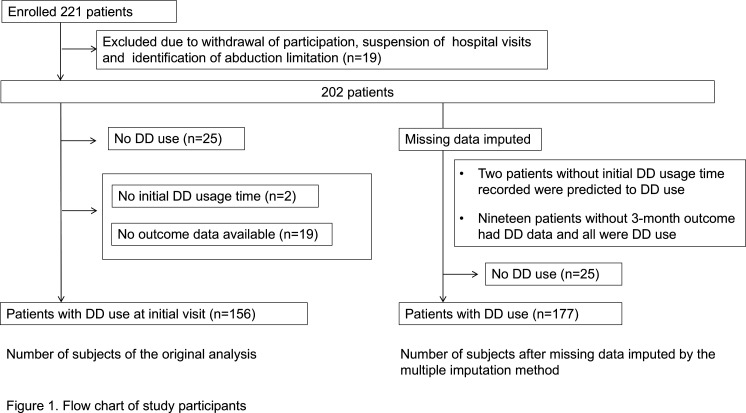
From the initial enrolment of 221 patients, 19 were excluded due to withdrawal of consent, lack of hospital visits before the study conclusion, or identification of abduction restrictions. Additionally, 25 patients who used DD for less than 60 min/day (for elementary school students and younger) and less than 120 min/day (for junior high school students and older) at the time of their initial examination were excluded from the study because they were considered unaffected by DD. Patients were also excluded if there were no data available on initial DD time (n=2) or outcomes (n=19), and finally 156 patients were included in the analysis (figure 1).
Figure 1. Flow chart of study participants. In total, 156 patients with DD use at initial visits were originally analysed. For the analysis using multiple imputation for missing data, 177 patients were included. DD, hand-held digital devices.
Data and measurement collection
We collected the following data: age, sex, medical history of strabismus or amblyopia, psychological stress, time from symptom onset to diagnosis (within 3 months), refractive values of both eyes, anisometropia exceeding 1.50 D, high myopia of −6.0 D or less, glasses or contact lens correction status, strabismus angle measured with the alternate prism cover test at distance (5 m) and near (33 cm), near stereoacuity, DD time, viewing distance when using DD and adherence to taking breaks while using DD.
Outcome groups
The criteria for determining the outcome were as follows: (1) cured: strabismus within 8 prism dioptres (PD) at both distance and near, with no subjective symptoms; (2) improved: subjective or objective improvement (strabismus angle within 8 PD, improvement of 10 PD or more, or maintained phoria); (3) unchanged: strabismus angle increased or decreased by less than 10 PD, or no improvement observed in subjective symptoms; and (4) worsened: strabismus angle increased by 10 PD or more or worsening of subjective symptoms. In cases of ambiguity in the determination of the outcome, the attending doctor’s perception was prioritised, followed by objective findings.
Statistical analysis
We conducted a univariate analysis to compare patient characteristics across outcome groups. Subsequently, we performed multivariate analysis using ordinal logistic regression, adjusted for several confounders, and further performed the analysis for multiple-imputed data. Continuous variables are presented as the mean (SD) or median (range), while categorical data are presented as the number of cases (%). For near stereopsis, statistical calculations were performed after logarithmic transformation, with no stereopsis as 10 000; results were then converted back to the antilogarithm and presented. Multiple group comparisons were performed using Fisher’s exact test, Friedman test and Kruskal-Wallis tests with post-hoc Holme or Steel-Dwass test. We performed a hierarchical cluster analysis with Ward’s method, using the DD times of the three visits to cluster patients based on different DD time change patterns. Ordinal logistic regression analysis was performed using variables representing changes in DD time and clinical data as explanatory variables to identify the factors associated with the outcome. We created models that were adjusted for stereopsis (model 1), distance strabismus angle (model 2) and success in halving DD time (model 3), with age as a covariate.
A sensitivity analysis was performed using the same approach on data with imputed missing values. Missing data were imputed using multiple imputation methods and predicted using all the variables of 202 patients who underwent the initial test. We created 20 imputed datasets and combined them using Rubin’s rule for another logistic regression on 177 patients with DD use (figure 1). Statistical analyses were performed using R (The R Foundation for Statistical Computing, Vienna, Austria) and its graphical user interface, EZR (Saitama Medical Centre, Jichi Medical University, Saitama, Japan).12 All tests were two-tailed, with statistical significance set at p<0.05.
Patient and public involvement statement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this study.
Results
In total, 156 patients (mean age (SD): 16.4 (7.4) years old, 88 males) were classified into the cured (n=10, 6.4%), improved (n=58, 37.2%), unchanged (n=67, 42.9%) and worsened (n=21, 13.5%) groups. The baseline esotropia angle of all patients was 25 (0, 70) PD at distance and 20 (0, 70) at near, with a decrease to 20 (−10, 60) PD (p=0.002) and 18 (−6, 66) PD (p=0.04) at 3 months, respectively. Table 1 shows the background characteristics and comparisons across the four outcome groups.
Table 1. Patient data by outcome groups.
| Variables/groups | Cured(n=10) | Improved(n=58) | Unchanged(n=67) | Worsened(n=21) | P value | |
| Age (years) | 15.0 (7, 23) | 16.0 (5, 33) | 15.0 (6, 35) | 16.0 (7, 31) | 0.90 | |
| 14.5 (6.4) | 16.6 (7.3) | 16.4 (7.9) | 16.4 (7.1) | |||
| Sex | Female | 5 (50) | 23 (40) | 32 (48) | 8 (38) | 0.74 |
| Male | 5 (50) | 35 (60) | 35 (52) | 13 (62) | ||
| History of strabismus or amblyopia | No | 9 (90) | 52 (90) | 61 (91) | 19 (91) | 1.00 |
| Yes | 1 (10) | 6 (10) | 6 (9) | 2 (9) | ||
| Emotional stress | No | 6 (60) | 35 (63) | 45 (67) | 12 (63) | 0.93 |
| Yes | 4 (40) | 21 (37) | 22 (33) | 7 (37) | ||
| Duration from onset to visit | Within 3M | 7 (70) | 11 (19) | 15 (22) | 8 (38) | 0.006 |
| More than 3M | 3 (30) | 46 (81) | 52 (78) | 13 (62) | ||
| Refractive error (D) | Right eye | −2.75(−8.75, 0.50) | −3.25(−8.62, 4.75) | −2.88(−16.50, 3.38) | −1.19(−10.00, 2.88) | 0.96 |
| −3.12 (3.27) | −2.65 (3.13) | −2.89 (3.79) | −2.96 (3.91) | − | ||
| Left eye | −2.50(−9.25, 0.50) | −3.00(−8.25, 5.00) | −2.75(−15.88, 3.88) | −0.50(−10.00, 5.75) | 0.77 | |
| −3.30 (3.19) | −2.61 (3.01) | −2.71 (3.65) | −2.11 (4.18) | − | ||
| Anisometropia 1.5 D or greater | No | 10 (100) | 55 (95) | 63 (94) | 20 (95) | 1.00 |
| Yes | 0 (0) | 3 (5) | 4 (6) | 1 (5) | ||
| Under correction of myopia 1.0 D or greater | No | 6 (60) | 42 (72) | 51 (76) | 16 (76) | 0.72 |
| Yes | 4 (40) | 16 (28) | 16 (24) | 5 (24) | ||
| Under correction of hyperopia 1.5 D or greater | No | 10 (100) | 56 (97) | 62 (93) | 20 (95) | 0.87 |
| Yes | 0 (0) | 2 (3) | 5 (7) | 1 (5) | ||
| High myopia −6.0 D or less | No | 7 (70) | 49 (85) | 58 (87) | 14 (67) | 0.12 |
| Yes | 3 (30) | 9 (15) | 9 (13) | 7 (33) | ||
| Using prism | No | 7 (70) | 45 (78) | 56 (84) | 16 (76) | 0.61 |
| Yes | 3 (30) | 13 (22) | 11 (16) | 5 (24) | ||
| Strabismus angle (PD) | ||||||
| Distance | Initial visit | 11 (6, 30) | 25 (0, 70) | 25 (4, 52) | 30 (10, 45) | 0.003 |
| Near | 12 (6, 35) | 18 (6, 70) | 20 (0, 50) | 25 (0, 53) | 0.30 | |
| Distance | 3 months | 3 (−10, 6) | 18 (2, 60) | 25 (4, 45) | 40 (12, 55) | <0.001 |
| Near | 3 (−6, 8) | 14 (0, 60) | 20 (0, 50) | 35 (10, 66) | <0.001 | |
| Stereoacuity (arcsec) | 50 (40, 400) | 200 (40, nil) | 400 (20, nil) | 1550 (40, nil) | 0.018 | |
| DD time (minutes) | 183 (60, 420) | 227 (60, 806) | 240 (69, 746) | 214 (60, 386) | 0.47 | |
| DD night-time use | No | 5 (50) | 30 (52) | 35 (52) | 14 (67) | 0.66 |
| Yes | 5 (50) | 28 (48) | 32 (48) | 7 (33) | ||
| Viewing distance30 cm or more | No | 9 (90) | 45 (80) | 55 (87) | 16 (80) | 0.69 |
| Yes | 1 (10) | 11 (20) | 8 (13) | 4 (20) | ||
Data are presented as mean (standard deviationSD), median [(range]), or number of cases (%).
DD, hand-held digital devices; DD timethe amount of time spent using hand-held digital devices per daynil, no detectable stereoacuity; PD, prism dioptres
In the univariate analysis, significant differences were observed in the number of patients who visited the hospital within 3 months of onset, angle of distance strabismus and stereoacuity. The median strabismus angle at distance in the cured group was 11 PD, which was significantly smaller than that in the other three groups (improved: 25 PD, p<0.01; unchanged: 25 PD, p<0.05; worsened: 30 PD, p<0.01). The median near stereoacuity of the cured group was 50 arcsec, significantly better than those of the unchanged and worsened groups (400 arcsec, p<0.05, and 1550 arcsec, p<0.01, respectively). Spectacles were prescribed for 42 (27%) patients for appropriate refractive correction, whereas prism glasses were already used by 10 (6.4%) patients at the initial visit and were newly prescribed to 22 (14%) patients. Prism glasses were prescribed with ground-in or Fresnel membrane prisms. No significant differences were observed in refractive values, undercorrection of hyperopia or myopia, anisometropia, or high myopia and the proportion of prism users in the four outcome groups.
Regarding the comparison of DD usage during the initial visit, no significant differences were observed among the four groups in terms of DD time, whether they were used at night, or whether the viewing distance was 30 cm away (table 1). Cluster analysis, categorising patients based on changes in DD time over three visits, showed that patients who reduced their DD time (clusters 1 and 2) typically achieved a reduction of more than half (online supplemental figure 1) Therefore, we defined successful DD time halving as cases in which the DD time after 1 or 3 months was less than half of the initial DD time, serving as a variable for change in DD time. Subsequently, we compared the DD usage conditions at the third-month visit and found no significant differences among the four groups in terms of whether they took breaks, maintained a distance of at least 30 cm, maintained the recommended time for use and achieved successful halving of DD time (Table 2).
Table 2. DD usage condition at 3 months by outcome groups.
| Variables/groups | Cured(n=10) | Improved(n=58) | Unchanged(n=67) | Worsened(n=21) | P value |
| Took breaktime when using DD (%) | |||||
| No | 1 (10) | 21 (39) | 26 (41) | 10 (50) | 0.19 |
| Yes | 9 (90) | 33 (61) | 37 (59) | 10 (50) | |
| Viewing distance 30 cm or more (%) | |||||
| No | 1 (10) | 9 (16) | 16 (25) | 8 (40) | 0.14 |
| Yes | 9 (90) | 46 (84) | 47 (75) | 12 (60) | |
| Adhering to the recommended time (%) | |||||
| No | 5 (50) | 29 (54) | 37 (61) | 13 (68) | 0.66 |
| Yes | 5 (50) | 25 (46) | 24 (39) | 6 (32) | |
| Successful DD time halving | |||||
| No | 3 (30) | 23 (40) | 32 (52) | 12 (63) | 0.21 |
| Yes | 7 (70) | 34 (60) | 30 (48) | 7 (37) | |
Data are presented as number of cases (%).
DD, hand-held digital devicesDD timethe amount of time spent using hand-held digital devices per day
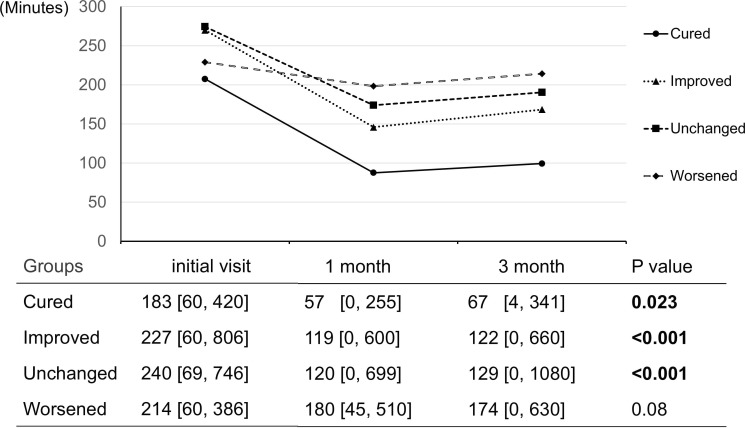
However, in terms of DD time, at 1 and 3 months, the cured, improved, and unchanged groups showed a significant decrease in DD time (cured: p=0.023, improved: p<0.001, unchanged: p<0.001), whereas the worsened group showed no decrease in DD time (p=0.08) (figure 2). Table 3 shows the results of the ordinal logistic analysis, with the outcomes ordered as cured, improved, unchanged and improved (see online supplemental table S1 for the full table). The analysis revealed that the outcomes were influenced by small-angle strabismus at distance (p=0.023), good stereoacuity (p=0.003) and successful DD time halving (p=0.016). The results of the same regression analysis performed after imputing missing data were similar to those of the original analysis (online supplemental table S2).
Figure 2. Change in DD time by outcome group. The line graphs show the change in the mean DD time at the initial visit, at 1 month, and at 3 months by outcome group. The table below shows the median (range) of the DD time and the Friedman test p value. DD, hand-held digital devices.
Table 3. Factors associated with the outcome analysed by ordinal logistic regression analysis using original data.
| Factors | Model 1 | Model 2 | Model 3 | |||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Sex (male) | 1.00 | 0.70 to 1.42 | 1.06 | 0.75 to 1.50 | 1.02 | 0.72 to 1.47 |
| Stereoacuity (log) | 1.22 | 0.99 to 1.51 | 1.31 | 1.10 to 1.56 | ||
| Adhering to the recommended time | 0.79 | 0.54 to 1.14 | 0.78 | 0.54 to 1.13 | 0.93 | 0.62 to 1.40 |
| Successful DD time halving | 0.63 | 0.43 to 0.92 | 0.63 | 0.43 to 0.92 | ||
| Strabismus angle at distance (PD) | 1.01 | 0.99 to 1.02 | 1.02 | 1.00 to 1.03 | ||
| Strabismus angle at near (PD) | 1.00 | 0.99 to 1.02 | 0.99 | 0.97 to 1.02 | 1.01 | 1.00 to 1.02 |
| Duration from onset to visit within 3 months | 0.93 | 0.62 to 1.38 | 0.94 | 0.63 to 1.41 | 0.86 | 0.57 to 1.28 |
| Refractive error (D) | 1.00 | 0.94 to 1.06 | 1.00 | 0.94 to 1.06 | 0.99 | 0.93 to 1.05 |
| Anisometropia 1.5 D or greater | 1.16 | 0.53 to 2.52 | 1.19 | 0.55 to 2.57 | 0.90 | 0.39 to 2.04 |
| Using prism | 0.97 | 0.63 to 1.50 | 0.93 | 0.61 to 1.44 | 0.84 | 0.55 to 1.30 |
| History of strabismus or amblyopia | 0.81 | 0.45 to 1.46 | 0.90 | 0.51 to 1.61 | 1.03 | 0.57 to 1.83 |
Model 1: Aadjusted for Aage and Sstereoacuity. Model 2: Aadjusted for age and strabismus angle at distance. Model 3: Aadjusted for age and successful DD time halving.
DdioptresDD timethe amount of time spent using hand-held digital devices per dayPDprism dioptres
Discussion
In this study, we aimed to investigate factors associated with outcomes after 3 months of instructed usage of DD and appropriate spectacle wear. Of 156 patients, 10 (6%), 58 (37%), 67 (43%) and 21 (13.5%) were in the cured, improved, unchanged and worsened group, respectively. Factors associated with good outcomes were small angle esotropia, good stereoacuity and successful halving of the time spent using DD.
Regarding the change in DD time during the follow-up period, a decrease was observed in the cured, improved and unchanged groups, whereas no decrease was observed in the worsened group. Additionally, ordinal logistic regression showed that failure to reduce DD time by less than half of the original time was associated with worse outcomes. These results indicate that limiting the use of DD is expected to improve esotropia, whereas continued use may worsen the condition.
Some previous studies910 13,15 have shown an improvement in strabismus angle after restricting smartphone use. Lee et al9 reported that cessation of smartphone use improved esotropia in all 12 cases and reduced the strabismus angle by approximately 10 PD, from a mean of 27.75 to 17.50 PD at distance fixation. Other studies showed that reducing the use of DD improved esotropia and diplopia in 5 out of 1510 and 4 out of 10 cases,15 respectively. In contrast, one study16 found no improvement in esotropia with reduced smartphone use. Therefore, the effectiveness of this approach is controversial. Although the mechanism of esotropia development from smartphone use is not fully understood, it has been suggested that the viewing distance is shorter when using a smartphone than when viewing hardcopy text,15 17 and that excessive accommodation and convergence occur,2 5 10 15 as well as the absence of distance viewing (without divergence eye movement).18 We speculate that reducing the amount of time spent using smartphones will reduce this abnormal viewing condition and, in some cases where the esotropia is in a reversible stage, may influence improvement. In cases with a small strabismus angle, limiting the use of DD may be particularly beneficial, since a reduction of 10 PD may lead to a cure.
In this study, cluster analysis was useful in examining trends in the changes in DD time, which are expected to vary from case to case in terms of compliance. Notably, the factor of successful DD time halving, rather than the recommended time by age, was associated with the outcome (table 3, onlinesupplemental tables S1 S2). This may reflect individual differences in the sensitivity to the effects of DD usage on esotropia.7 19
Other factors associated with positive outcomes in this study were good stereoacuity and small-angle strabismus at distance. Stereo test results are influenced by the ocular position.20 Specifically, with a smaller strabismus angle, it is easier to maintain esophoria and better stereo test results can be expected. Regardless of the duration of DD usage, stereoacuity and strabismus angle were considered important prognostic factors for the outcomes in this study.
Regarding the relationship between the effectiveness of instructional DD usage and the duration from onset, while it has been reported that the strabismus angle is more likely to decrease in shorter periods since onset,13 non-reduction in the strabismus angle even within just 1 month after onset has been reported.16 In our study, a higher proportion of patients in the cured group presented within 3 months of symptom onset. However, this variable was not a significant factor in the multivariate analysis.
Concerning the classification criteria for the four outcomes, the inclusion of the change in subjective symptoms as a criterion may have introduced ambiguity in the classification decision. However, because some patients with a small strabismus angle from the initial examination were included, subjective symptoms were necessary to determine outcomes. Moreover, the change in strabismus angle was determined by 10 PD; however, the clinical significance of the change in strabismus angle differed depending on the original size of the angle. In cases with a large original esotropia angle, even a 10 PD reduction in the strabismus angle had less clinical significance; the improved group included such cases. Therefore, although a relationship between reduction in the use of DD by half and esotropia outcome was observed, we suggest that it is important to establish preventive strategies because it is difficult to cure esotropia with a large angle once it has developed.
Strengths and limitations
The strength of our study lies in the numerical analysis based on the diaries of the DD time. This allowed us to investigate the relationship between outcomes and varying DD usage time due to compliance issues. However, this study had a few limitations. First, DD time relied on self-reporting by the patients and their guardians, introducing potential inaccuracies. Second, some clinical data and questionnaires contained missing data. However, the robustness of the results is demonstrated by the fact that sensitivity analysis using multiple imputation methods showed consistent results. Finally, this study did not define the criteria for prescribing prism glasses. Although an active treatment method using gradual prism reduction for patients with AACE with a small strabismus angle has been reported,18 the present study could not fully investigate the effect of prism use on outcome. Therefore, further research is needed to clarify the criteria for prescribing a relieving or a neutralising prism and to evaluate the efficacy of the prism.
Conclusions
In patients with ACE using DD at onset, providing instructions on DD usage resulted in cure in 6% patients and in improvement in 37% patients. Positive outcomes were associated with a small-angle esotropia, good stereoacuity and successful halving of DD time. Therefore, in patients using DD at onset, it may be worthwhile to attempt to halve the time of DD use first.
supplementary material
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Funding: This work was supported by JSPS KAKENHI (grant JP20K0978), a grant from the National Centre for Child Health and Development (30-2330-23 and 2021B-6), and a grant for the multicentre study from the Japanese Association for Strabismus and Amblyopia.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study was approved by the Ethics Committee of Hamamatsu University Medical School (19-091), the Central Ethics Review Board of the National Centre for Child Health and Development (2019-016) and the ethical review board of the individual participating sites. Written informed consent, outlining the nature and potential consequences of the study, was obtained from each patient or their guardians if they were below 20 years of age. This study adhered to the tenets of the Declaration of Helsinki. Participants gave informed consent to participate in the study before taking part.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Data availability free text: Deidentified participant data supporting the findings of this study are available from the corresponding author MS on reasonable request.
Contributor Information
Noriko Nishikawa, Email: nnori@asahikawa-med.ac.jp.
Hirohito Iimori, Email: crgx240@gmail.com.
Reiko Kinouchi, Email: rkino@asahikawa-med.ac.jp.
Sachiko Nishina, Email: nishina-s@ncchd.go.jp.
Tomoyo Yoshida, Email: uemura-t@ncchd.go.jp.
Akiko Hikoya, Email: ahikoya@hama-med.ac.jp.
Miwa Komori, Email: komori@hama-med.ac.jp.
Osamu Hieda, Email: ohieda@koto.kpu-m.ac.jp.
Toshiaki Goseki, Email: goseki@iuhw.ac.jp.
Takafumi Mori, Email: t-mori@fmu.ac.jp.
Takeshi Morimoto, Email: takeshi.morimoto@ophthal.med.osaka-u.ac.jp.
Takashi Negishi, Email: tnegishi@juntendo.ac.jp.
Tamami Shimizu, Email: ttamami1102@gmail.com.
Yukiko Shimizu, Email: Y.shimizu@tsukazaki-eye.net.
Shion Hayashi, Email: hayashi-sh@ncchd.go.jp.
Yoshiko Sugiyama, Email: yosh0823@mx.bw.dream.jp.
Yoshimi Yokoyama, Email: yoshimi@chukyogroup.jp.
Akiko Kimura, Email: akikoshimmyo@gmail.com.
Hiroko Suzuki, Email: ohishihiroko@gmail.com.
Sadao Suzuki, Email: ssuzuki@med.nagoya-cu.ac.jp.
Noriyuki Azuma, Email: azuma-nkt@yacht.ocn.ne.jp.
Miho Sato, Email: mihosato@hama-med.ac.jp.
Data availability statement
Data are available upon reasonable request.
References
- 1.BURIAN HM, MILLER JE. Comitant convergent strabismus with acute onset. Am J Ophthalmol. 1958;45:55–64. doi: 10.1016/0002-9394(58)90223-x. [DOI] [PubMed] [Google Scholar]
- 2.Hoyt CS, Good WV. Acute onset concomitant esotropia: when is it a sign of serious neurological disease? Br J Ophthalmol. 1995;79:498–501. doi: 10.1136/bjo.79.5.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu M, Tang Y, Wang Z, et al. Clinical characteristics and risk factors of acute acquired concomitant esotropia in last 5 years: a retrospective case–control study. Eye (Lond) 2023;37:320–4. doi: 10.1038/s41433-022-01939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roda M, Pellegrini M, Rosti A, et al. Augmented bimedial rectus muscles recession in acute acquired concomitant esotropia associated with myopia. Can J Ophthalmol. 2021;56:166–70. doi: 10.1016/j.jcjo.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Topcu Yilmaz P, Ural Fatihoglu Ö, Sener EC. Acquired Comitant Esotropia in Children and Young Adults: Clinical Characteristics, Surgical Outcomes, and Association With Presumed Intensive Near Work With Digital Displays. J Pediatr Ophthalmol Strabismus. 2020;57:251–6. doi: 10.3928/01913913-20200422-02. [DOI] [PubMed] [Google Scholar]
- 6.Okita Y, Kimura A, Masuda A, et al. Yearly changes in cases of acute acquired comitant esotropia during a 12-year period. Graefes Arch Clin Exp Ophthalmol. 2023;261:2661–8. doi: 10.1007/s00417-023-06047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim CW, Lee J, Kim WJ. Changes in the number and characteristics of patients with acute acquired concomitant esotropia over time: An 8-year retrospective study. Medicine (Balt) 2023;102:e33986. doi: 10.1097/MD.0000000000033986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roda M, di Geronimo N, Valsecchi N, et al. Epidemiology, clinical features, and surgical outcomes of acute acquired concomitant esotropia associated with myopia. PLoS ONE. 2023;18:e0280968. doi: 10.1371/journal.pone.0280968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HS, Park SW, Heo H. Acute acquired comitant esotropia related to excessive Smartphone use. BMC Ophthalmol. 2016;16:37. doi: 10.1186/s12886-016-0213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neena R, Remya S, Anantharaman G. Acute acquired comitant esotropia precipitated by excessive near work during the COVID-19-induced home confinement. Indian J Ophthalmol. 2022;70:1359–64. doi: 10.4103/ijo.IJO_2813_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iimori H, Nishina S, Hieda O, et al. Clinical presentations of acquired comitant esotropia in 5-35 years old Japanese and digital device usage: a multicenter registry data analysis study. Jpn J Ophthalmol. 2023;67:629–36. doi: 10.1007/s10384-023-01023-5. [DOI] [PubMed] [Google Scholar]
- 12.Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. B M T. 2013;48:452–8. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi R, Hayashi S, Machida S. The effects of topical cycloplegics in acute acquired comitant esotropia induced by excessive digital device usage. BMC Ophthal. 2022;22:366. doi: 10.1186/s12886-022-02590-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta A, Greensher JE, Dahl GJ, et al. Acute Onset Esotropia From Excessive Smartphone Use in a Teenager. J Pediatr Ophthalmol Strabismus. 2018;55:e42–4. doi: 10.3928/01913913-20181017-01. [DOI] [PubMed] [Google Scholar]
- 15.Van Hoolst E, Beelen L, De Clerck I, et al. Association between near viewing and acute acquired esotropia in children during tablet and smartphone use. Strabismus. 2022;30:59–64. doi: 10.1080/09273972.2022.2046113. [DOI] [PubMed] [Google Scholar]
- 16.Mohan A, Sen P, Mujumdar D, et al. Series of cases of acute acquired comitant esotropia in children associated with excessive online classes on smartphone during COVID-19 pandemic; digital eye strain among kids (DESK) study-3. Strabismus. 2021;29:163–7. doi: 10.1080/09273972.2021.1948072. [DOI] [PubMed] [Google Scholar]
- 17.Bababekova Y, Rosenfield M, Hue JE, et al. Font size and viewing distance of handheld smart phones. Optom Vis Sci. 2011;88:795–7. doi: 10.1097/OPX.0b013e3182198792. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Feng X, Li J, et al. Prismatic treatment of acute acquired concomitant esotropia of 25 prism diopters or less. BMC Ophthal. 2022;22:276. doi: 10.1186/s12886-022-02501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iimori H, Suzuki H, Komori M, et al. Clinical findings of acute acquired comitant esotropia in young patients. Jpn J Ophthalmol. 2022;66:87–93. doi: 10.1007/s10384-021-00879-9. [DOI] [PubMed] [Google Scholar]
- 20.Read JCA. Stereo vision and strabismus. Eye (Lond) 2015;29:214–24. doi: 10.1038/eye.2014.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.