Extended Data Fig. 5 ∣. MTBD and MTBH1-2 optimally position the TTLL6 core to interact with the negatively charged α-tubulin tail through a groove lined with positively charged residues critical for activity.
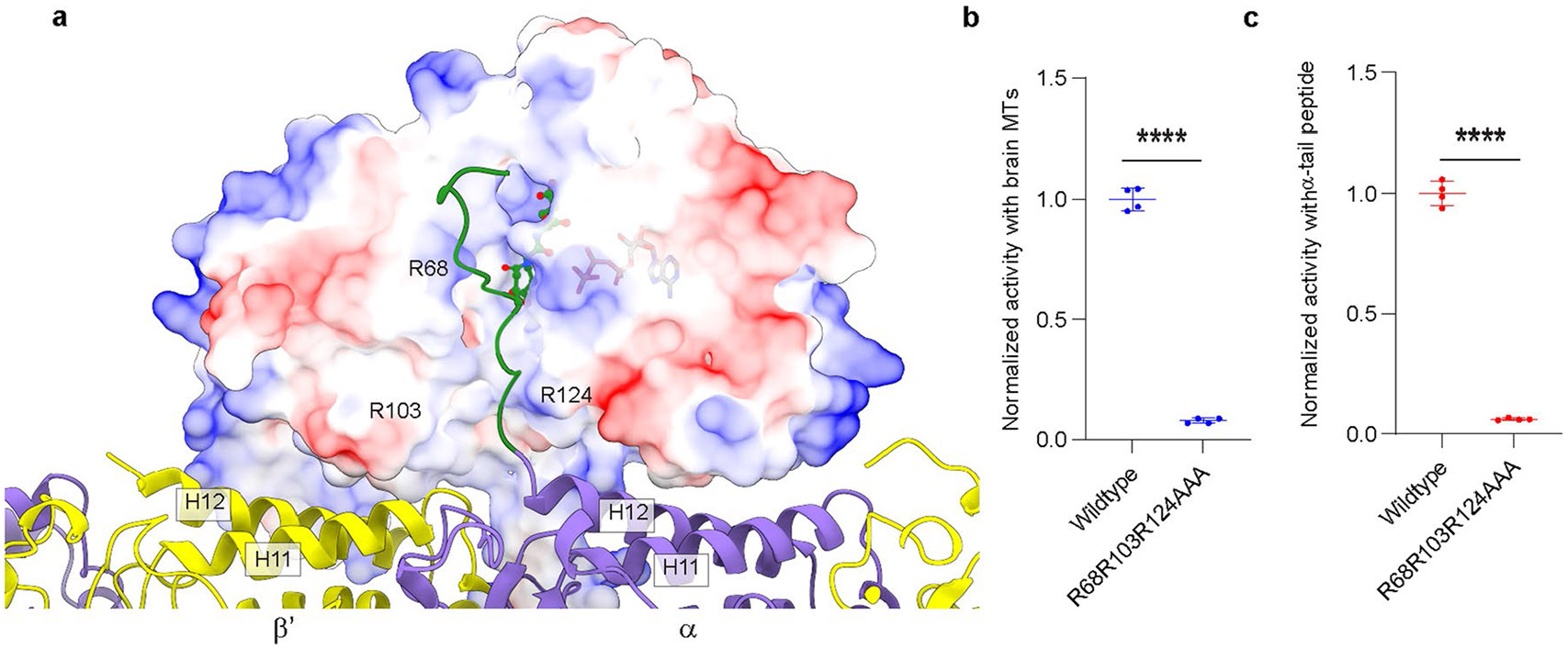
a. View of TTLL6 in complex with the microtubule showing the proximity of the C-terminal tail of α-tubulin to the active site of TTLL6. TTLL6 surface is color-coded for electrostatic potential. The electrostatic potential was calculated using the Poisson Boltzmann Solver77. The α-tubulin tail, shown in green, for which no structural data are available was modeled to maximize side chain interactions without violating stereochemical constraints. ATP and the di-glutamate in the active site are shown in ball-and-stick. The di-glutamate position is based on the X-ray crystal structure of TTLL6 in complex with the α-elongation analog (PDB 6VZU; ref. 41). A groove lined with positively charged residues leads to the active site of TTLL6. We hypothesize that these cationic residues anchor electronegative side chains in the α-tubulin tail, while the hydrophobic groove interacts with the tubulin tail backbone. b,c. Normalized glutamylation activity of structure-guided TTLL6 mutants in the proposed α-tubulin tail binding groove with taxol-stabilized microtubules (b) and isolated α1B(-Y) peptide (c). Error bars, S.E.M. (n = 4). ****p < 0.0001 as determined by two-tailed t-test.
