Extended Data Fig. 2 ∣. MTBH1-2 is highly flexible and binds only to microtubules and not soluble tubulin.
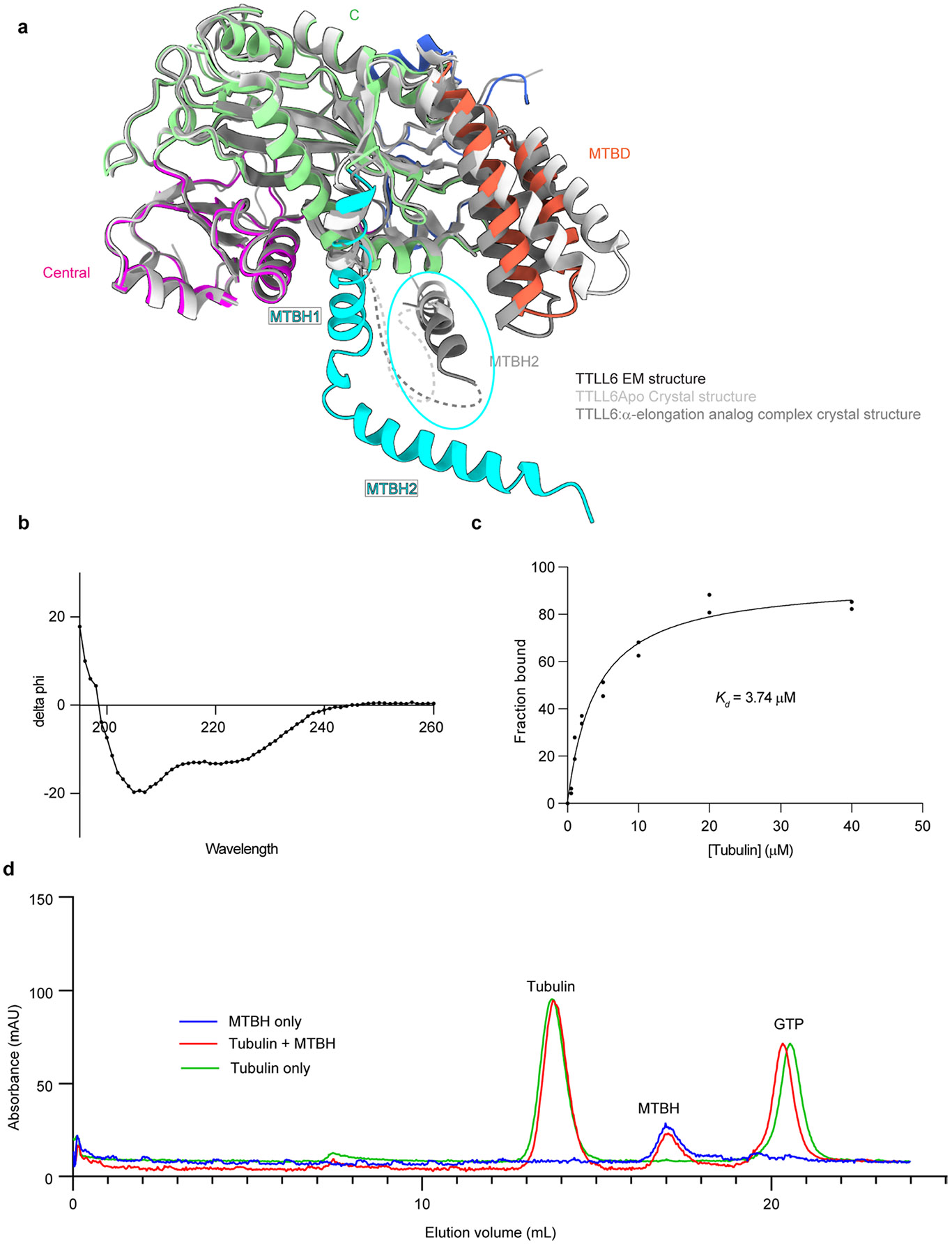
a. Ribbon representation of TTLL6 in the conformation it adopts in complex with the microtubule (domains colored as in Fig. 1) superposed on the X-ray crystal structures of TTLL6 bound to ATP (light gray, PDB 6VZT; ref. 41) or bound to a di-Glu elongation analog (dark gray, PDB 6VZU; ref. 41). A part of the MTBH1-2 was not resolved in the X-ray structures. The unresolved regions are shown as dotted lines. The MTBH1-2 in the TTLL6 X-ray structures, highlighted by a cyan ellipse, is in a different orientation than that in the cryo-EM structure in complex with the microtubule. b. Circular dichroism spectra of recombinantly expressed and purified TTLL6 MTBH1-2 show its secondary structure is predominantly α-helical. c. Binding of TTLL6 MTBH1-2 to taxol-stabilized microtubules assembled from porcine brain tubulin. Kd ~3.7 μM. Error bars, S.E.M (n = 2). d. Gel filtration analysis of recombinant TTLL6 MTBH1-2 and soluble αβ-tubulin mixture showing that the two proteins elute separately on a Superdex-200 analytical gel-filtration column (Methods).
