Abstract
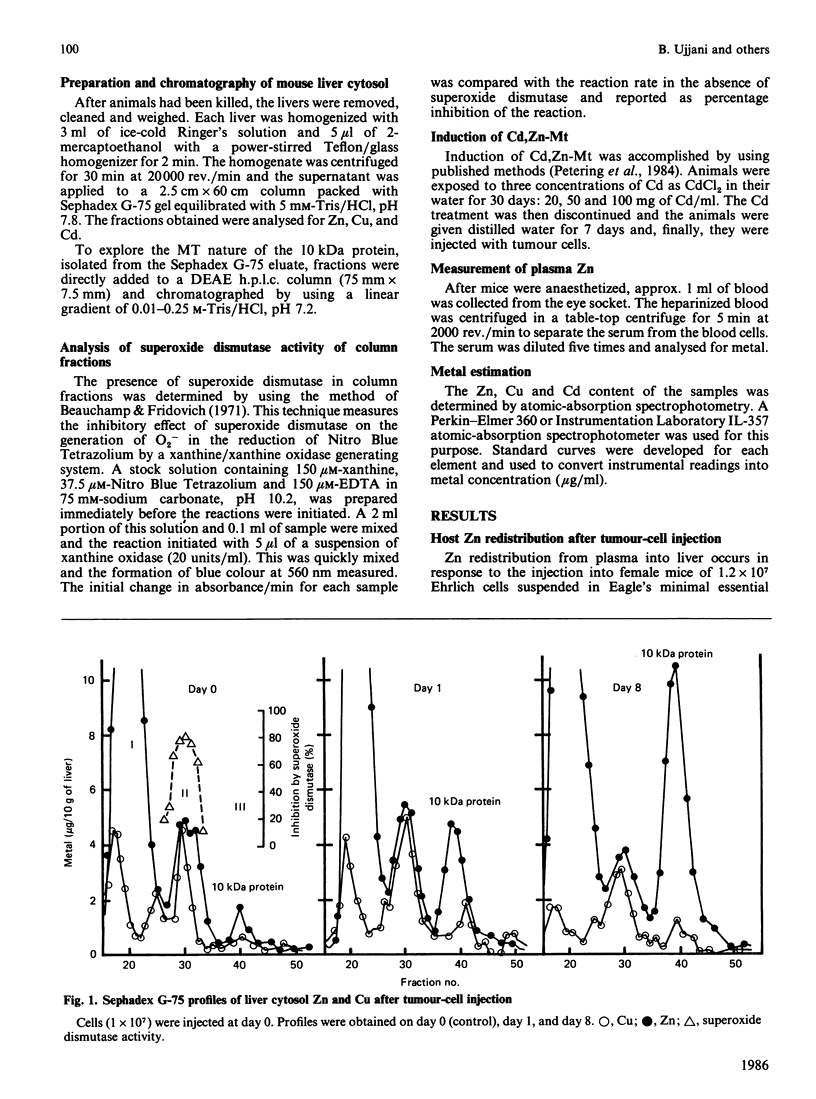
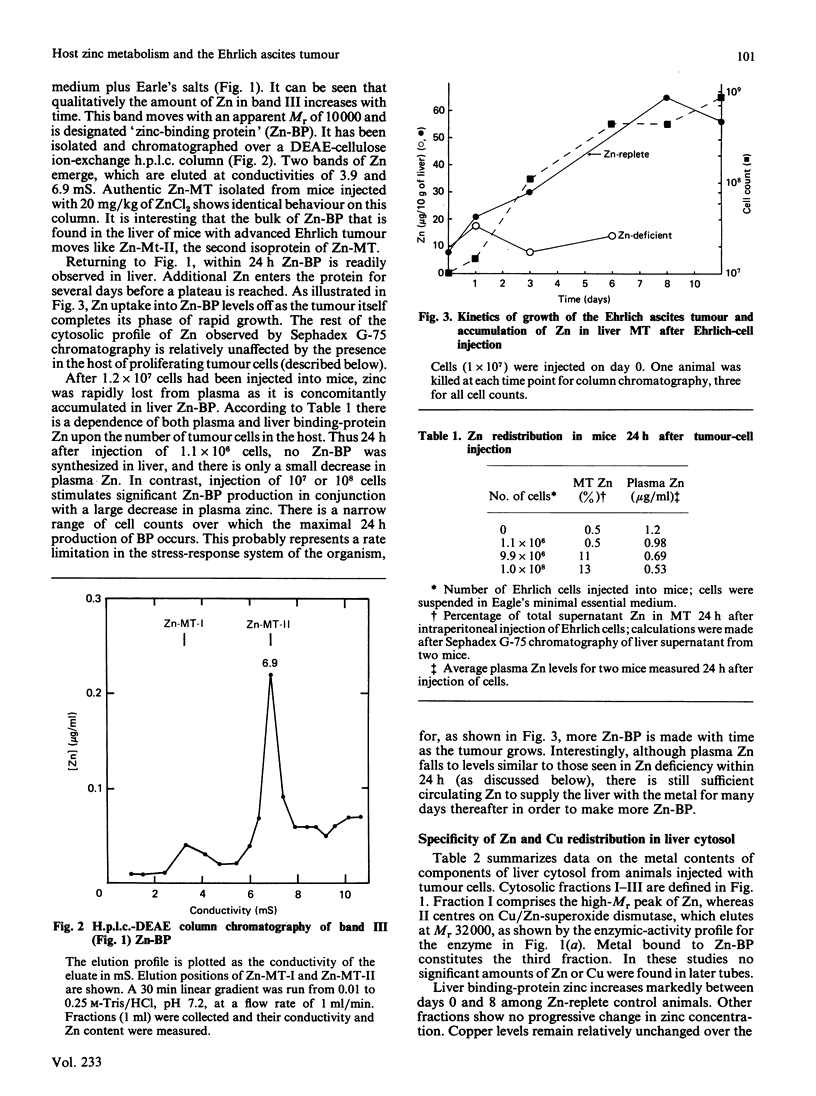
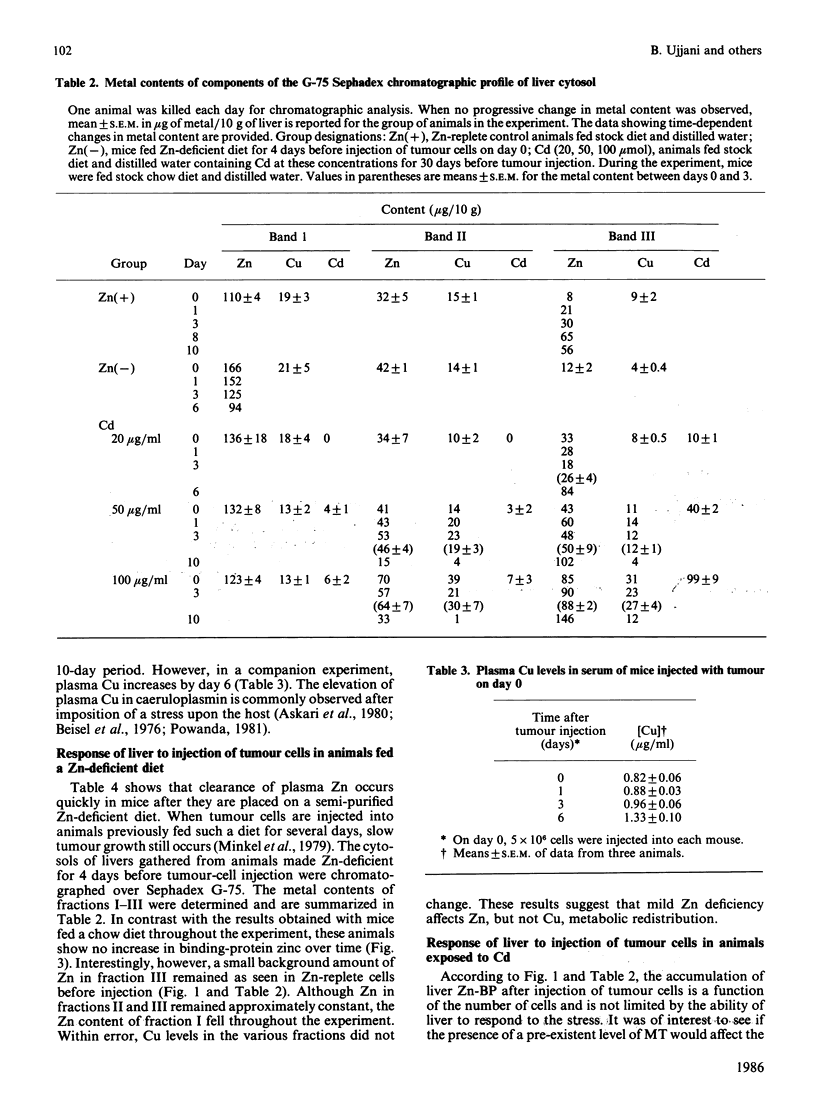
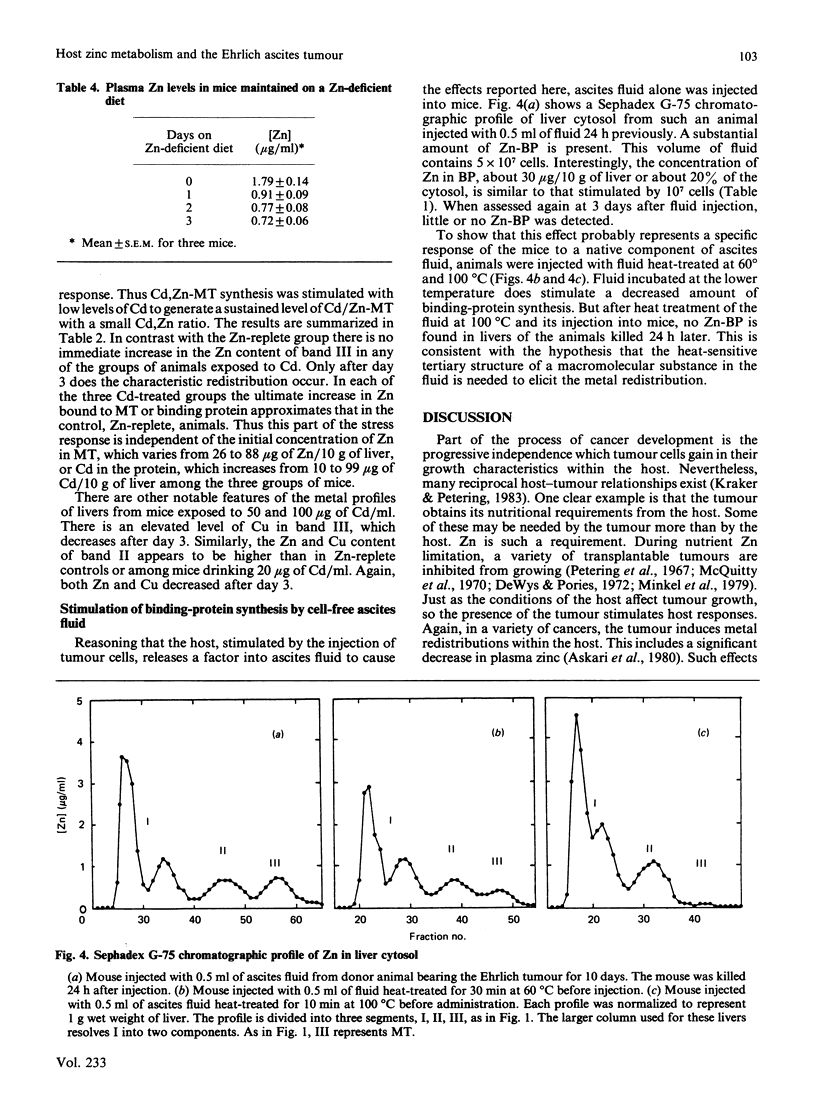
Zinc redistribution between plasma and liver has been examined in mice injected with Ehrlich-ascites-tumour cells. Within 24 h of injection plasma Zn levels decrease and Zn appears in newly synthesized liver metallothionein. This response is dependent upon the number of tumour cells injected into the host. Uptake of Zn into liver and its specific accumulation in a Zn-binding protein, identified as metallothionein, continues for a number of days and reaches a plateau as tumour growth ceases. Over this time period, plasma copper rises. This redistribution also occurs in mice pretreated with cadmium in their drinking water for 1 month at levels of 20, 50, and 100 micrograms/ml. However, in each case there is a lag of 3 days before Zn increases in the livers of these animals which already contain substantial amounts of Cd/Zn-metallothionein. When Ehrlich cells are injected into mice previously placed on a Zn-deficient diet for several days, plasma Zn is already low and no net uptake of Zn into liver metallothionein is apparent. Finally, it is shown that ascites fluid can itself stimulate a transient shift of host of Zn into liver. Heat-inactivated fluid loses this property. It is suggested that, in the peritoneum, tumour cells initiate a stress response mediated by an ascites-fluid factor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askari A., Long C. L., Blakemore W. S. Zinc, copper, and parenteral nutrition in cancer. A review. JPEN J Parenter Enteral Nutr. 1980 Nov-Dec;4(6):561–571. doi: 10.1177/0148607180004006561. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- DeWys W., Pories W. Inhibition of a spectrum of animal tumors by dietary zinc deficiency. J Natl Cancer Inst. 1972 Feb;48(2):375–381. [PubMed] [Google Scholar]
- Feldman S. L., Cousins R. J. Degradation of hepatic zinc-thionein after parenteral zinc administration. Biochem J. 1976 Dec 15;160(3):583–588. doi: 10.1042/bj1600583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampschmidt R. F. Leukocytic endogenous mediator. J Reticuloendothel Soc. 1978 Apr;23(4):287–297. [PubMed] [Google Scholar]
- Lachman L. B. Human interleukin 1: purification and properties. Fed Proc. 1983 Jun;42(9):2639–2645. [PubMed] [Google Scholar]
- McQuitty J. T., Jr, DeWys W. D., Monaco L., Strain W. H., Rob C. G., Apgar J., Pories W. J. Inhibition of tumor growth by dietary zinc deficiency. Cancer Res. 1970 May;30(5):1387–1390. [PubMed] [Google Scholar]
- Minkel D. T., Dolhun P. J., Calhoun B. L., Saryan L. A., Petering D. H. Zinc deficiency and growth of Ehrlich ascites tumor. Cancer Res. 1979 Jul;39(7 Pt 1):2451–2456. [PubMed] [Google Scholar]
- Minkel D. T., Poulsen K., Wielgus S., Shaw C. F., 3rd, Petering D. H. On the sensitivity of metallothioneins to oxidation during isolation. Biochem J. 1980 Nov 1;191(2):475–485. doi: 10.1042/bj1910475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S. H., Deagen J. T., Whanger P. D., Weswig P. H. Biological function of metallothionein. V. Its induction in rats by various stresses. Am J Physiol. 1978 Mar;234(3):E282–E285. doi: 10.1152/ajpendo.1978.234.3.E282. [DOI] [PubMed] [Google Scholar]
- Ohtake H., Hasegawa K., Koga M. Zinc-binding protein in the livers of neonatal, normal and partially hepatectomized rats. Biochem J. 1978 Sep 15;174(3):999–1005. doi: 10.1042/bj1740999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake H., Koga M. Purification and characterization of zinc-binding protein from the liver of the partially hepatectomized rat. Biochem J. 1979 Dec 1;183(3):683–690. doi: 10.1042/bj1830683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Stadler B. M., Siraganian R. P., Mage M., Mathieson B. Lymphokines: their role in lymphocyte responses. Properties of interleukin 1. Fed Proc. 1982 Feb;41(2):257–262. [PubMed] [Google Scholar]
- Otvos J. D., Armitage I. M. Structure of the metal clusters in rabbit liver metallothionein. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7094–7098. doi: 10.1073/pnas.77.12.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petering D. H., Loftsgaarden J., Schneider J., Fowler B. Metabolism of cadmium, zinc and copper in the rat kidney: the role of metallothionein and other binding sites. Environ Health Perspect. 1984 Mar;54:73–81. doi: 10.1289/ehp.845473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petering H. G., Buskirk H. H., Crim J. A. The effect of dietary mineral supplements of the rat on the antitumor activity of 3-ethoxy-2-oxobutyraldehyde bis(thiosemicarbazone). Cancer Res. 1967 Jun;27(6):1115–1121. [PubMed] [Google Scholar]
- Sas B., Bremner I. Effect of acute stress on the absorption and distribution of zinc and on Zn-metallothionein production in the liver of the chick. J Inorg Biochem. 1979 Aug;11(1):67–76. doi: 10.1016/s0162-0134(00)80055-0. [DOI] [PubMed] [Google Scholar]
- Sobocinski P. Z., Canterbury W. J., Jr, Mapes C. A., Dinterman R. E. Involvement of hepatic metallothioneins in hypozincemia associated with bacterial infection. Am J Physiol. 1978 Apr;234(4):E399–E406. doi: 10.1152/ajpendo.1978.234.4.E399. [DOI] [PubMed] [Google Scholar]
- Webb M., Cain K. Functions of metallothionein. Biochem Pharmacol. 1982 Jan 15;31(2):137–142. doi: 10.1016/0006-2952(82)90202-7. [DOI] [PubMed] [Google Scholar]


