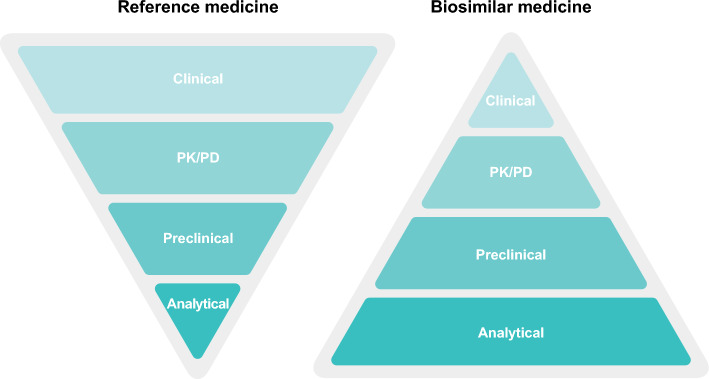
Fig. 1.
The totality of evidence data package for the development and approval of biosimilar medicines compared to reference medicines. In vivo preclinical studies are not a requirement from the European Medicines Agency and US Food and Drug Administration for the approval of biosimilar medicines when extensive analytical and functional characterization has already demonstrated the proposed biosimilar and reference medicines to be highly similar [9, 22, 38]. PD pharmacodynamics, PK pharmacokinetics