Fig. 4.
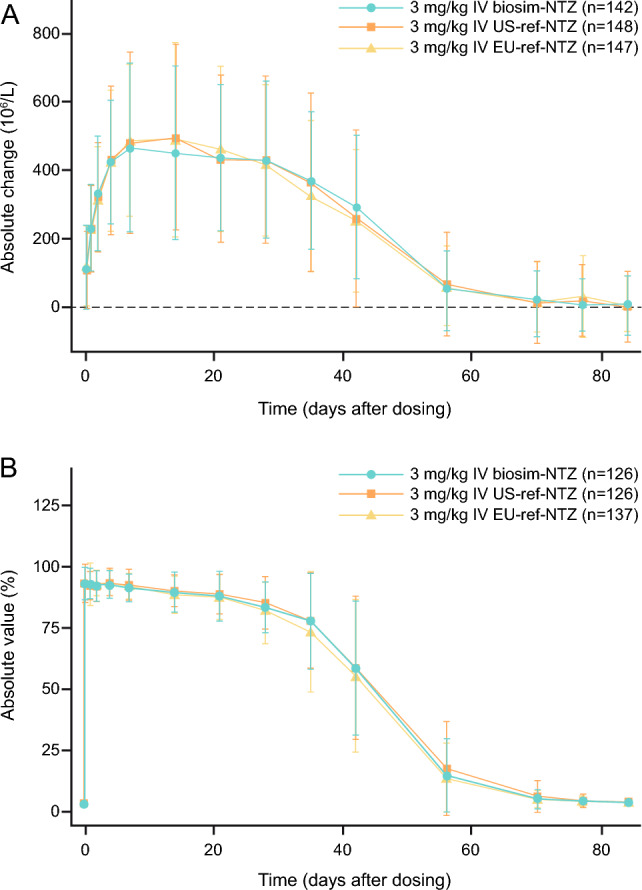
Primary pharmacodynamic endpoints (engagement set). A Change from baseline in mean (SD) CD19+ cell counts over time for biosimilar natalizumab (biosim-NTZ) versus USA (US)-reference natalizumab (ref-NTZ) and European Union (EU)-ref-NTZ. B Change from baseline in mean (SD) α4-integrin percentage receptor saturation (RS) over time for biosim-NTZ versus US-ref-NTZ and EU-ref-NTZ. IV intravenous, PD pharmacodynamic. Wessels et al. [49]. Reprinted by permission of Informa UK Limited, trading as Taylor & Francis Group, https://www.tandfonline.com
