Figure 2.
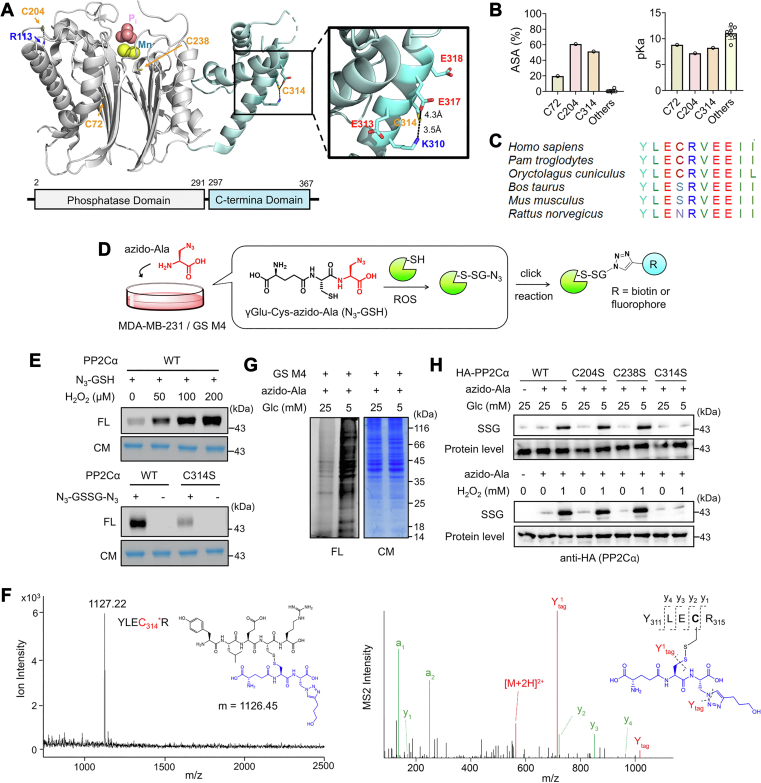
PP2Cα is susceptible to glutathionylation at non-conserved Cys 314.A, PP2Cα structure. A model shows phosphate (Pi) and Mn2+ in the active site and locations of three relatively surface-exposed cysteines (C72, C204, and C314). B, comparisons of pKa and accessible surface area (ASA) for cysteines in PP2Cα. C, the amino acid sequence alignment around C314 among different species, showing limited conservation of C314. D, a scheme of a clickable glutathione approach for analyzing S-glutathionylation. Cells expressing GS M4 are incubated with azido-Ala, which enables biosynthesis of clickable glutathione (azido-glutathione, N3-GSH). N3-GSH forms S-glutathionylation in oxidative stress. Alternatively, purified protein can form glutathionylation in the presence of N3-GSH, which is analyzed after the click reaction. E, glutathionylation of purified PP2Cα in vitro. Purified PP2Cα was incubated with N3-GSH and H2O2 for 15 min (top) or oxidized azido-glutathione (N3-GSSG-N3) for 30 min (bottom). PP2Cα glutathionylated by azido-glutathione was conjugated with rhodamine-alkyne via click chemistry and visualized by Coomassie stain (CM, protein level) and fluorescence (FL, SSG level) (n = 3). F, MALDI-TOF and MS/MS analyses of glutathionylated peptides in PP2Cα. Purified PP2Cα glutathionylated by azido-glutathione was click-conjugated by biotin-DADPS-alkyne, enriched by streptavidin-agarose, and digested by trypsin on beads. Glutathionylated peptides were eluted and analyzed by MALDI-TOF (left) and LC-MS/MS (right), finding a glutathionylated peptide at C314 (YLEC314∗R, m/z 1127). In the MS2 spectrum, all y ions were found with additional ions (Y1tag and Ytag) resulting from fragmentation in glutathione modification (n = 3). G, global glutathionylation in MDA-MB-231. MDA-MB-231 cells expressing GS M4 (MDA-MB-231/GS M4) were stimulated in low or high glucose conditions. After the click reaction of lysates with rhodamine-alkyne, proteins were analyzed by fluorescence and Coomassie stain (n = 3). H, PP2Cα glutathionylation in MDA-MB-231. MDA-MB-231/GS M4 cells expressing HA-PP2Cα WT or C/S mutant were incubated in low or high glucose conditions for 24 h or with H2O2 for 15 min. After the click reaction of lysates with biotin-alkyne, glutathionylated PP2Cα was probed by Western blot before (protein level) and after (SSG) enrichment by streptavidin-agarose (n = 3). Data are representative of three independent experiments.