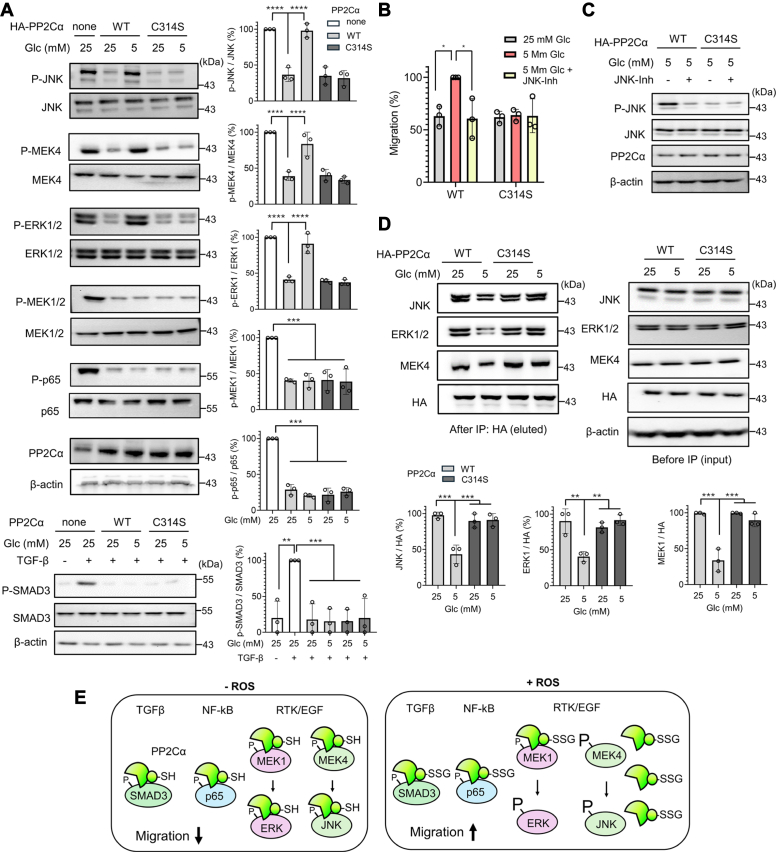
Figure 4.
PP2Cα C314 glutathionylation activates JNK and ERK pathways by selectively altering protein–protein interactions.A, activation of JNK, ERK1/2, and MEK4 upon PP2Cα glutathionylation. MDA-MB-231 cells expressing PP2Cα WT or C314S were incubated in low or high glucose for 6 h, and phosphorylation levels of PP2Cα substrates were analyzed by western blots (n = 3). B–C, JNK activation is responsible for increased cell migration. The wound-healing migration for 24 h (n = 3) (B) and JNK phosphorylation after 6 h (n = 3) (C) in cells expressing PP2Cα WT or C314S upon the addition of JNK-inhibitor (JNK-Inh). D, PP2Cα glutathionylation induces its dissociation from JNK, ERK1/2, and MEK4. After incubation of cells in low or high glucose for 6 h, co-immunoprecipitation was used to monitor their binding interactions by western blots (n = 3). E, a model for cell migration induced by PP2Cα glutathionylation. In a nonstressed condition, PP2Cα binds to its substrates for their dephosphorylation, which suppresses cell migration. However, H2O2 or ROS induced by low glucose causes PP2Cα glutathionylation at C314, which dissociates PP2Cα from selected signaling substrates, such as JNK, ERK1/2, and MEK4. The dissociation increases phosphorylation levels of JNK, ERK1/2, and MEK4, which causes increased cell migration via the JNK-paxillin pathway. Data show the mean ± SD or representative of three independent experiments. The statistical difference was analyzed by one-way ANOVA with Tukey’s post hoc test (A, D) or two-way ANOVA with Turkey’s post hoc test (B), where ∗p < 0.03, ∗∗p < 0.002, ∗∗∗p < 0.0002, ∗∗∗∗p < 0.0001.