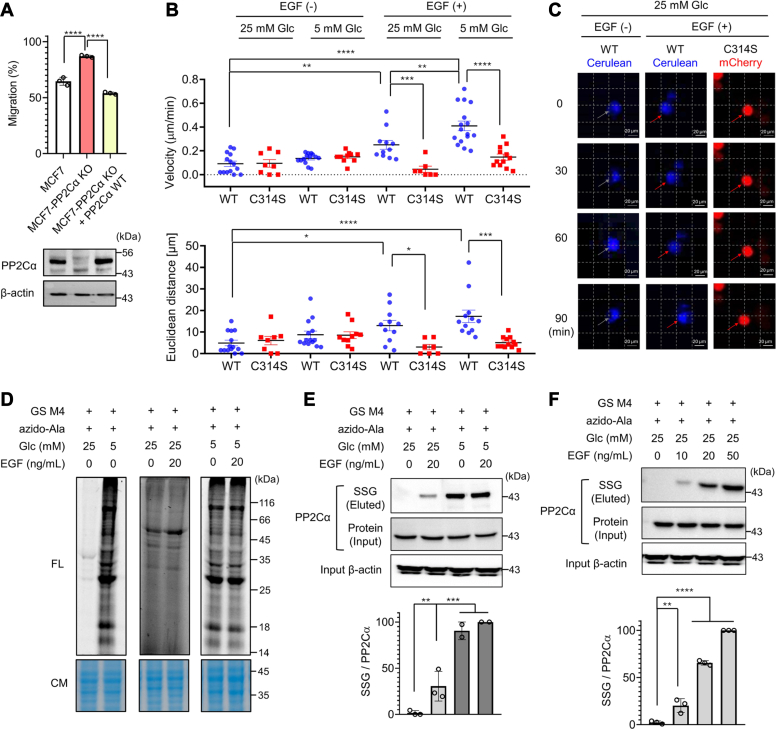
Figure 5.
PP2Cα C314 glutathionylation constitutes growth factor–mediated redox signaling.A, migration of MCF7-PP2Cα KO cell lines without and with ectopic expression of PP2Cα. The wound-healing migration of MCF7-derived cell lines in high glucose (25 mM) for 24 h (top) and PP2Cα levels in the cell lines (bottom) (n = 3). B–C, migration velocities and distances of individual MCF7-PP2Cα KO cells expressing PP2Cα WT (MCF7-PP2Cα WT) or C314S (MCF7-PP2Cα C314S). MCF7-PP2Cα WT with Cerulean (blue) and MCF7-PP2Cα C314S with mCherry (red) were combined and incubated in low or high glucose without or with EGF for 1.5 h. B, individual cells (n = 7–14) were monitored and analyzed for migration distances and velocities. C, representative images of individual cell migration. The scale bar represents 20 μm. D–F, EGF induces PP2Cα glutathionylation in redox signaling. Global glutathionylation (n = 3) (D) and PP2Cα glutathionylation (n = 3) (E–F) in MCF7 upon adding EGF for 16 h. After incubation of EGF, lysates were click-conjugated with rhodamine-alkyne (D) or biotin-alkyne (E–F). Glutathionylated proteins were analyzed by fluorescence (FL, SSG levels) with Coomassie stains (CM, protein levels) (D) or western blots after (SSG level) and before (protein level) enrichment by streptavidin-agarose (E–F). Data show the mean ± SD or representative of three independent experiments. The statistical difference was analyzed by one-way ANOVA and Tukey’s (A, B, E) or Dunnett’s (F) post hoc test, where ∗p < 0.03, ∗∗p < 0.002, ∗∗∗p < 0.0002, ∗∗∗∗p < 0.0001.