Abstract
Autism is a neurodevelopmental disorder distinguished by impaired social interaction and repetitive behaviors. Global estimates indicate that autism affects approximately 1.6% of children, with the condition progressively becoming more prevalent over time. Despite noteworthy progress in autism research, the condition remains untreatable. This serves as a driving force for scientists to explore new approaches to disease management. Autism is linked to elevated levels of oxidative stress and disturbances in the DNA repair mechanism, which may potentially play a role in its comorbidities development. The current investigation aimed to evaluate the beneficial effect of the naturally occurring flavonoid proanthocyanidins on the behavioral characteristics and repair efficacy of autistic BTBR mice. Moreover, the mechanisms responsible for these effects were clarified. The present findings indicate that repeated administration of proanthocyanidins effectively reduces altered behavior in BTBR animals without altering motor function. Proanthocyanidins decreased oxidative DNA strand breaks and accelerated the rate of DNA repair in autistic animals, as evaluated by the modified comet test. In addition, proanthocyanidins reduced the elevated oxidative stress and recovered the disrupted DNA repair mechanism in the autistic animals by decreasing the expressions of Gadd45a and Parp1 levels and enhancing the expressions of Ogg1, P53, and Xrcc1 genes. This indicates that proanthocyanidins have significant potential as a new therapeutic strategy for alleviating autistic features.
Keywords: Autism, Antioxidants, DNA strand breaks, DNA repair, Tumor
1. Introduction
Autism is an array of diverse neurodevelopmental conditions identified as autism spectrum disorder that typically shows during the first three years of life. It is described by difficulties in social interactions and communication (Hodges et al., 2020). Autism frequently coexist with intellectual disability and congenital malformations, both of which are linked to a higher likelihood of developing cancer (Liu et al., 2022a, Liu et al., 2022b). The incidence of autism is increasing, likely as a result of increased awareness and diligent screening. The incidence of autism has been estimated to be 1.69 %, with a boy-to-girl ratio of approximately 4 to 1 (de Giambattista et al., 2021). Once signs of autism manifest, individuals require ongoing support throughout their whole lives, and their life expectancy is decreased due to the presence of multiple comorbidities.
The cause of autism is unclear, although genetic influences and environmental effects, comprising social determinants, seem to elevate the likelihood of developing autism. Polymorphisms in DNA repair genes are commonly associated with autism, and there is a clear link between mutations in DNA damage/repair genes and autism (Dasdemir et al., 2016, Markkanen et al., 2016). Disturbance of repair mechanisms can impair the aptitude of cells to repair themselves and lead to the increase of DNA strand breaks. The majority of DNA strand breaks are promptly repaired due to the presence of DNA repair enzymes. Nevertheless, if DNA damage occurs to genes that produce DNA repair enzymes, the cell's capacity to repair itself is diminished. Thus, errors will build up in other genes, facilitating DNA damage and, consequently, cancer development (Madabhushi et al., 2014). Elevated levels of oxidative DNA strand breaks and impaired DNA repair efficiency are observed in rodent models of autism and diagnosed autistic individuals (Shpyleva et al., 2014, Markkanen et al., 2016, Attia et al., 2020a, Attia et al., 2020b).
Disturbance in repairing DNA strand breaks makes the individual more susceptible to substances that can damage DNA and potentially contribute to cancer development. Autism is frequently linked to an increased risk of developing tumors, and patients with autism have a higher occurrence of CNS, ovarian, and breast malignancies compared to those without autism (Kao et al., 2010, Crespi, 2011). The suggested elucidation for the elevated risk of cancer in autistic patients is the impaired DNA repair mechanisms. Previous studies have recognized a substantial overlap in the genes associated with cancer and autism and many of these genes are involved in repairing damaged DNA, suggesting that the elevated probability of tumor development in patients with autism may primarily be driven by genetic factors (Crespi, 2011, Crawley et al., 2016).
Autistic patients often use behavioral therapy and serotonin-specific reuptake inhibitors to treat the characteristic behaviors associated with autism. Nevertheless, ongoing research is exploring alternative treatments in response to the limited effectiveness of current therapies. Moreover, it is crucial to enhance the deficient DNA repair capacity in patients with autism to restore the DNA molecules that have been damaged and to prevent the onset of malignant consequences. Given the circumstances, we predicted that grape seed proanthocyanidins, natural flavonoids, could be beneficial due to their demonstrated efficacy in protecting against DNA damage and cancer (Mancini et al., 2023). This is particularly noteworthy because they possess antioxidant capabilities and can regulate DNA damage and repair pathways. In addition, proanthocyanidins demonstrate neuroprotective properties in many neurodegenerative and neuropsychiatric disorders (Duarte et al., 2023). Scientific evidence has demonstrated that grape seed proanthocyanidins possess antidepressant properties, enhance memory function, and reduce anxiety-like behavior caused by increased oxidative stress (Allam et al., 2013). Additionally, proanthocyanidins can reduce neuroinflammation, enhance cognitive function, and alleviate most of the neuropathological characteristics associated with neurodegenerative disorders (Chen et al., 2018, Duarte et al., 2023).
Several studies have demonstrated the strange biological effects of grape seed proanthocyanidins, including antioxidant, anti-cancer, anti-inflammatory, antimicrobial, anti-diabetic, anti-obesity, anti-neurodegenerative, and cardio- and eye-protective properties (Nie et al., 2023). Despite their strong effectiveness, proanthocyanidins demonstrate minimal toxicity in mouse safety testing (Yamakoshi et al., 2002). Given that proanthocyanidins are both safe and commercially available natural antioxidants, it is imperative to investigate their impact on autism. The therapeutic effects of proanthocyanidins in autism have not been well studied, and there is limited data on their effectiveness in treating autistic symptoms (Arafat and Shabaan, 2019, Mahdipour et al., 2023). The extract demonstrated a notable improvement in the markers of oxidative stress, as well as the histological and morphometric characteristics of the cerebellar cortex in autistic rats, due to the potent antioxidant impact of the extract.
While these limited data do not provide definitive evidence of the therapeutic potential of proanthocyanidins against this disorder, they demonstrate that proanthocyanidins have ameliorative effects against autism, suggesting that they could be an effective therapeutic strategy. Nevertheless, further research is necessary to examine the impact of proanthocyanidins on various aspects of autism. Thus, the objective of the present investigation was to assess the influence of proanthocyanidins on behavioral characteristics and DNA repair efficiency in a mouse model of autism. Furthermore, the specific mechanism(s) responsible for the ameliorative properties of proanthocyanidins were clarified. We utilized BTBR T + Itpr3tf/J (BTBR) mice in this investigation due to their immune system characteristics and behavioral features, including impaired social interactions, elevated repetitive self-grooming, and limited communications, which are similar traits observed in autistic subjects. Thus, BTBR mice are a pertinent model for autism research (Meyza and Blanchard, 2017, Kisaretova et al., 2023). The age-matched C57BL/6J (B6) inbred strain mice were used as control animals, because of their unique social tendencies, which have been widely studied for their behavioral characteristics in social interactions.
2. Materials and methods
2.1. Animals
The investigation utilized male BTBR mice aged 5–7 weeks (Jackson Laboratories, Bar Harbor, US). To maintain consistency with the higher prevalence of autism in human males, we exclusively used male mice in our studies to exclude the potential impact of hormones in female animals. The control group consisted of B6 inbred strain mice, aged 5–7 weeks, procured from the Experimental Animal Care Center at King Saud University. We kept the mice in a controlled environment, maintaining a temperature of around 22 °C and a relative humidity of about 50 %. We subjected the mice to a 12-hour light/dark cycle, allowing them unrestricted access to regular pellets and drinking water. Throughout our investigation, we kept the animals in plastic boxes, each containing five mice, in an adequately ventilated animal house. The Institutional Animal Care and Use Committee of King Saud University has approved these investigations (KSU-SE21-64).
2.2. Experimental design
Once the animals acclimatized to the animal house, they were divided into five groups, each including eight mice. Group 1 comprised B6 animals that were administered daily oral distilled water via oral intubations as a vehicle for four weeks. The group was categorized as the normal control mice. Group 2 comprised BTBR animals that received daily oral administration of distilled water via oral intubations as a vehicle for four weeks. Group 3 comprised B6 animals that received a daily oral administration of proanthocyanidins (Spectrum Chemical Mfg. animals, CA, USA) by oral intubations at 100 mg/kg/day for four weeks. Group 4: BTBR animals received daily oral intubation of proanthocyanidins at a dose of 100 mg/kg/day for four weeks. Group 5 comprised B6 animals that were administered a single intraperitoneal (i.p.) injection of 25 mg/kg of the positive control genotoxicant cyclophosphamide 24 h before euthanasia (Attia, 2012b, Attia, 2012a). Fig. 1 schematically represents the proanthocyanidins administration, behavioral assessments and scarification. The sample size in each experiment was chosen based on our previous experience with these tests. In studies of behavior, utilizing eight animals per group with a probability threshold of 0.05 (2-sided) allows obtaining statistical powers of 80 % for identifying differences between treated and untreated mice (Rosner 2011). The number of animals in the subsequent studies consisted of six mice per group. Following administering the treatments, the mice were provided unrestricted food and water until euthanized. The dose and duration of proanthocyanidins were selected based on the previous studies (Attia et al., 2010, Bakheet et al., 2016). If we compare the mice's dose to the equivalent human dose, 100 mg/kg of proanthocyanidins in mice would correspond to 8.1 mg/kg in humans, which corresponds to a 486 mg dose of proanthocyanidins for a 60 kg person. Consuming plants such as grape seed may not reasonably achieve this concentration, but taking a daily oral commercially available supplement (i.e., one capsule of 500 mg per day) can provide it.
Fig. 1.
A schematic drawing of the proanthocyanidins administration, behavioral assessments and scarification.
2.3. Behavioral studies
We performed behavioral assessments, such as the self-grooming test, marble burying test, sociability test, and open field test, 48 h prior to scarification. This study aims to examine the influence of proanthocyanidins on the behavioral problems displayed by BTBR animals. We conducted the behavior studies under low-light conditions, keeping skilled observers unaware of the treatments and groups. We used ordinary mouse cages for the spontaneous self-grooming test to measure repeated behavior. After 10-min accommodations, the duration of time that each mouse spent grooming body parts was measured for a 10-min period, as previously describe (Silverman et al., 2010). For the marble-burying behavior, we placed each animal in a cage corner and left them alone for 30 min. We placed a grid of four to five marbles, each measuring 15 mm in diameter, on top of 4 cm of normal bedding in the cage. At the end of the test, we counted the number of marbles each mouse had hidden (at least 50 %) (Attia et al., 2020a, Attia et al., 2020b).
In the 3-chamber sociability test, mouse was acclimated for 5 min in the center chamber before the start of the test. A control (stranger-1) mouse was positioned on each side of the compartment containing the wired cup container, and the testing mouse's social abilities were evaluated by timing how much time the mice spent in each chamber over ten min (session I). Furthermore, a second mouse (stranger-2) is placed inside the second wired cup to evaluate the testing mouse's social novelty. As mentioned in session I, the same parameters are watched for ten min. The test mouse's total time spent near both cups is divided by how much time it spends near the cup containing the novel mouse to get the social proximity index. The resulting number is then multiplied by 100. The social exploration domain can be determined by multiplying the results by 100 after dividing the test mouse's total time in all chambers by the time spent in the new mouse's chamber (Hussein et al., 2024). To assess the influence of proanthocyanidins on motor activity, mice underwent an open-field test to see whether the treatment had any sedative or motor function-altering effects. The apparatus consisted of a square floor surface with 50 × 50 cm dimensions. The floor was divided into 16 identical squares and surrounded by 40 cm-high walls on all sides. Following acclimatization, the animal was carefully located in a corner square, and the cumulative distance covered was counted for a five-minute interval as outlined previously (Silverman et al., 2010).
2.4. Biochemical and molecular studies
After 24 h of the behavioral tests, the animals were killed by cervical dislocation following inhalation of isoflurane (20 %). The femur and brain tissues were isolated, and femur bone marrow cells were flushed with saline into glass tubes. The brain tissues were washed with PBS, and the hippocampi were isolated and immediately used or stored by freezing them in liquid nitrogen at a temperature of −80 °C until needed.
2.4.1. Evaluation of spontaneous DNA strand breaks
The standard comet assay measures the frequency of single or double DNA strand breakage and labile alkali damage in an alkaline environment. The assay was carried out in accordance with the OECD guidelines as previously stated (Attia et al., 2010). Bone marrow cells were mixed with low-melting point agarose and smeared to a comet slide coated with regular-melting-point agarose. The slides were submerged in a cold lysis buffer for 12 h in a refrigerator. Subsequently, the slides were put into a gel electrophoresis chamber that contained a refrigerated electrophoresis buffer with a pH > 13. Then, the electrophoresis, neutralization and staining process were done. Two comet slides were made for each animal, and at least 150 well-dispersed cells were chosen from each slide for examination under a fluorescence microscope connected to the Comet Assay-IV computer software. We quantified the extent of DNA strand breaks by measuring the tail intensity, a percentage of the total DNA content in the comet tail.
2.4.2. Evaluation of oxidative DNA strand breaks
To evaluate the extent of DNA strand breaks caused by oxidation, the standard comet assay was modified by incorporating the repair enzymes Fpg and Endo III into the slide gel. Following cell lysis, the enzymes Fpg and Endo III were added to the gel, as previously detailed (Collins et al., 1993, Attia et al., 2013). Each slide was incubated at 37 ℃ for 30–45 min with either a diluted enzyme or an enzyme buffer. Then, the assay was performed using the same methodology as the standard comet test. To determine the total values of oxidative DNA strand breaks identified by Endo III and Fpg, we calculated the difference between the percentage of tail intensity observed when repair enzymes had been added (endogenous DNA strand breaks and oxidized bases) and the percentage of tail intensity observed in the absence of repair enzymes (only enzyme buffer, endogenous DNA strand breaks).
2.4.3. Evaluating the efficiency of DNA repair
To examine the kinetics of DNA repair, we performed the comet test at different time points following mice exposure to gamma radiation to induce DNA strand breaks. Two groups of animals, one receiving proanthocyanidins treatment and the other receiving solvent, were exposed to gamma radiation at a total dose of 4 Gy using a 60Co source (Gamma Cell-220, Nordion International Inc.) to induce DNA strand breaks (Al-Mazroua et al., 2019). Six mice from each irradiated group were euthanized at each specified time interval (0–––24 h) after exposure to gamma radiation. In addition, the experiment incorporated sham groups, which comprised B6 and BTBR mice. At specific times, animals were killed by cervical dislocation. Cells from the bone marrow of the femur were collected, and the prepared gels were lysed. Subsequently, they underwent electrophoresis, gel neutralizations, ethidium bromide staining, and scoring following the abovementioned procedure.
2.4.4. Evaluation of the redox balance
To assess the impact of proanthocyanidins on the interruption of oxidants and antioxidants balance in BTBR animals, the concentrations of reactive oxygen species (ROS) accumulation, reduced glutathione (GSH), and oxidized glutathione (GSSG) contents were measured 24 h following the final administration of proanthocyanidins. The formation of ROS was measured in bone marrow cells using DCFH2-DA, as detailed earlier (Bakheet et al., 2016). Hippocampus was homogenized in ice-cold 5 % potassium phosphate buffer. Following centrifugations, the supernatants were isolated, and the concentration of GSH was determined using DTNB according to the previously established method (Ellman 1959). GSSG evaluation was conducted using NADPH, glutathione reductase, and DTNB, based on the earlier protocol (Anderson 1985). The GSH and GSSG concentrations were calculated from standard curves of GSH and GSSG standard solutions and expressed as mM/mg protein. The quantity of GSH was divided by the concentration of GSSG to calculate the GSH/GSSG ratio (Attia 2012).
2.4.5. Gene expression analysis
To better understand how proanthocyanidins decrease DNA strand breaks and improve DNA repair capacity, the expression levels of some genes complicated with DNA damage and repair processes were determined in the hippocampus by real-time reverse transcription polymerase chain reaction (RT-PCR). The total RNA extraction procedure was performed using the TRIzol reagent (Invitrogen) according to the manufacturer's instructions (Attia et al., 2017). Complementary DNA was generated using a High-Capacity cDNA kit (Applied Biosystems), following the manufacturer's guidelines. The expression levels of Ogg1, Gadd45a, Parp1, Xrcc1, P53, and GAPDH in complementary DNA were measured using the listed primers (Table 1). The relative expression was calculated by the 2-ΔΔCT method (Livak and Schmittgen 2001).
Table 1.
Primers used in RT-PCR.
| Forward: 5′-3′ | Reverse: 5′-3′ | |
|---|---|---|
| P53 | 5′-CACAGCGTGGTGGTACCTTA-3′ | 5′-TCTTCTGTACGGCGGTCTCT-3′ |
| Gadd45a | 5′-CAGAGCAGAAGACCGAAAGG-3′ | 5′-GCAGGCACAGTACCACGTTA-3′ |
| Parp1 | 5′-GGAAAGGGATCTACTTTGCCG-3′ | 5′-TCGGGTCTCCCTGAGATGTG-3′ |
| Xrcc1 | 5′-CAGACAGCACACATCTCATC-3′ | 5′-ACCCTCCTCAGTTCATCCT-3′ |
| Ogg1 | 5′-GATTGGACAGTGCCGTAA-3′ | 5′-GGAAGTGGGAGTCTACAG-3′ |
| GAPDH | 5′-TGCCGCCTGGAGAAACC | TGAAGTCGCAGGAGACAACC |
2.5. Statistical analysis
All data are reported as mean ± SD. The results were firstly tested for homogeneity (Bartlett's and F tests) and normality (Kolmogorov and Smirnov test) before analyses with either the ANOVA test followed by Bonferroni's post-hoc test or the Kruskal-Wallis test followed by Dunn's post-hoc test for multiple comparisons (GraphPad Prism 5). Spearman rank correlation was employed to assess the relationships between molecular and behavioral data. The results were considered significant at P < 0.05.
3. Results
3.1. Proanthocyanidins mitigate behavioral alterations in BTBR animals
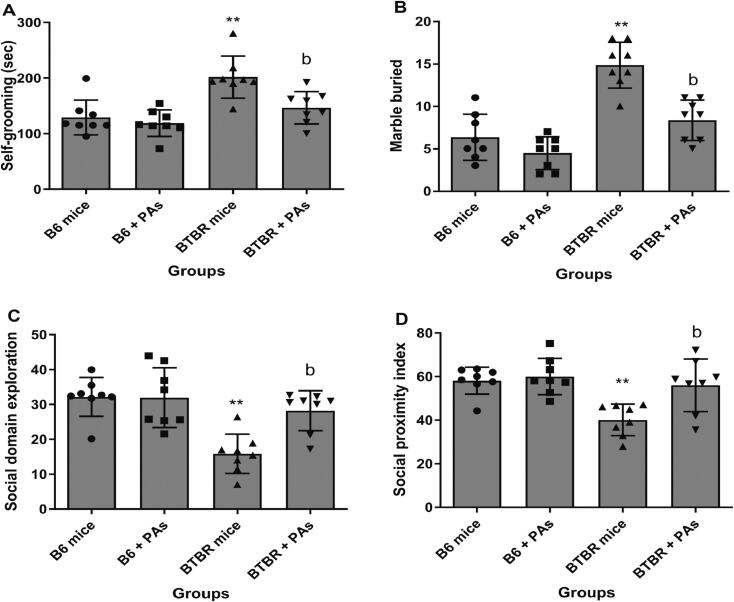
Fig. 2A–D illustrate that autistic animals displayed spontaneous self-grooming, higher marble-burying, and diminished sociality, indicative of autism-like behaviors. The administration of proanthocyanidins resulted in a notable decrease in buried marbles and spontaneous self-grooming while enhancing social interactions in the BTBR mice. The impacts were assessed by interaction analysis in the two-way ANOVA test. In B6 mice, proanthocyanidins did not affect these measures, and all the data were nearly similar to the control B6 mice. Fig. 2A shows that proanthocyanidins treatment effectively reduced the high self-grooming incidence in BTBR animals compared to BTBR mice that did not receive any treatment (P < 0.01). The main results showed that treatment (F(1, 28) = 8.915, P < 0.0058) and strain (F(1, 28) = 20.99, P < 0.0001) had significant effects. There was also a significant interaction between these two factors (F(1, 28) = 4.208, P < 0.0497). The Bonferroni post-hoc test showed that the treatment with proanthocyanidins had a significant effect, but this effect was only seen in the autistic strain of mice (BTBR mice: t = 3.562, P < 0.01; B6 animals: t = 0.6608, P > 0.05).
Fig. 2.
Effect of proanthocyanidins (PAs) administration on autism-like behaviors. (A) repetitive self-grooming, (B) marbles buried, (C) social domain exploration, and (D) social proximity index in B6 and BTBR animals (means ± SD, N = 8). **P < 0.01 vs. B6 animals; aP < 0.05 and bP < 0.01 vs. BTBR animals (Two-way ANOVA followed by Bonferroni post-hoc tests for multiple comparisons).
As shown in Fig. 2B, BTBR animals displayed significantly higher buried marbles than the B6 animals (P < 0.01). Administering proanthocyanidins to autistic animals led to a notable decrease in marble-burying incidences compared to the autistic mice not treated with proanthocyanidins (P < 0.05). There were statistically significant main impacts for strains (F(1, 28) = 50.86, P < 0.0001) and treatments (F(1, 28) = 23.29, P < 0.0001). Furthermore, there were significant interactions between the strains and treatment variables (F(1, 28) = 7.104, P < 0.0126). The Bonferroni post-hoc test demonstrated that proanthocyanidins treatment significantly influenced mice's BTBR strain. More precisely, the autistic mice displayed a substantial difference (t = 5.287, P < 0.001), while the B6 animals did not indicate any substantial differences (t = 1.528, P > 0.05).
In terms of sociability, autistic animals showed significantly less interest in exploring the social domains (Fig. 2C) and had a lower social proximity index (Fig. 2D) in comparison with the B6 mice (P < 0.01). The analysis of social domain exploration suggested that proanthocyanidins were likely linked to a significant increase in social domain exploration in treated autistic animals compared to untreated BTBR animals. There was a noteworthy main influence for treatment (F(1, 28) = 6.940, P < 0.0136) and strain (F(1, 28) = 19.03, P < 0.0002), along with a substantial interaction between both of those factors (F(1, 28) = 7.485, P < 0.0107). The Bonferroni post-hoc analysis demonstrated that the administration of proanthocyanidins had a substantial impact in autistic animals. In the B6 animals, proanthocyanidins had no significant effect (t = 0.07170, P > 0.05). However, in the autistic animals, it had a considerable influence (t = 3.797, P < 0.01).
Furthermore, proanthocyanidins treatment improved the social proximity index, which quantifies social interactions with foreign animals, in autistic mice (Fig. 2D). Nevertheless, proanthocyanidins did not influence these measures in B6 animals. There was a substantial main influence for treatment (F(1, 28) = 8.306, P < 0.0075) and strain (F(1, 28) = 12.70, P < 0.0013), along with a noteworthy interaction between these two components (F(1, 28) = 5.138, P < 0.0313). Post-hoc analysis revealed that the administration of proanthocyanidins had a statistically noteworthy impact, particularly in autistic animals. The treatments did not have a substantial effect on the B6 animals (t = 0.4352, P > 0.05), but they had a significant influence on the autistic animals (t = 3.641, P < 0.01).
The open-field test was conducted on all mice to assess the impact of proanthocyanidins treatment on motor function. No apparent variations in the ambulatory activity of any mice were found, irrespective of strain or treatment. Autistic animals displayed elevated locomotor activity levels relative to B6 animals solely during the first test period. Nevertheless, there was no notable variation in their total ambulatory frequency. The treatment of proanthocyanidins had no impact on the ambulatory activity of both B6 and BTBR mice, as indicated by the lack of a substantial difference in the entire number of crossed squares. The strain component did not exhibit any significant impact (F(1, 28) = 3.580, P = 0.0689). Likewise, the treatment component had no statistical influence (F(1, 28) = 0.07614, P = 0.3903). Additionally, the interaction between treatment and strain also did not have any significant effect (F(1, 28) = 0.3348, P = 0.5675) (two-way ANOVA test). The Bonferroni post-hoc testing indicated that the administration of proanthocyanidins did not have a statistically significant effect on either strain of mice (B6 mice: t = 0.2078, P > 0.05; BTBR mice: t = 11.026, P > 0.05).
3.2. Proanthocyanidins mitigate the occurrence of endogenous DNA strand breaks in BTBR animals
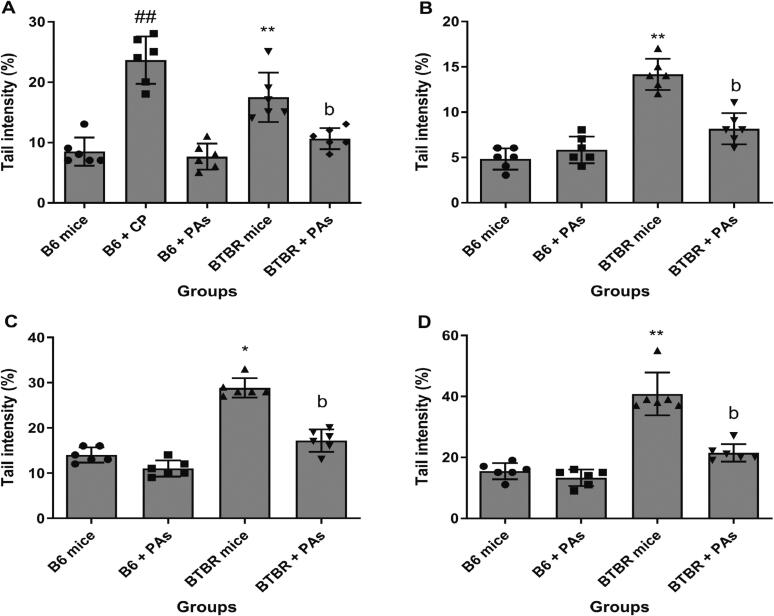
The data in Fig. 3A show that the incidence of DNA strand breaks in B6 mice treated with cyclophosphamide was significantly higher compared to B6 control animals (P < 0.01). This confirms that the comet assay is sensitive to accurately assessing DNA strand breakage. The B6 mice that received proanthocyanidins did not show any difference in the incidence of DNA strand breaks compared to the control B6 mice. Moreover, there was a 2.05-fold rise in endogenous DNA strand breaks in BTBR animals compared to B6 controls (P < 0.01). In contrast, BTBR animals that received proanthocyanidins exhibited a considerably lower incidence of DNA strand breaks than BTBR animals that did not (P < 0.05).
Fig. 3.
The levels of DNA strand break in the bone marrow cells of mice with and without proanthocyanidins treatments (mean ± SD, N = 6). The tail intensity % measures the degree of DNA strand breaks. (A) level of DNA strand breaks was assessed using the standard comet test. Oxidative DNA strand breaks were assessed by the modified comet test involving enzyme buffer incubation (B), Fpg repair enzyme incubation (C), and Endo III repair enzyme incubation (D). *P < 0.05, **P < 0.01 vs. the corresponding B6 control mice (Kruskal–Wallis test). bP < 0.01 vs. the corresponding untreated BTBR animals and ##P < 0.01 vs. B6 control animals (Mann–Whitney U test).
3.3. Proanthocyanidins reduce oxidative DNA strand breakage in BTBR mice
Incubating the control sides with DNA glycosylases dramatically increased the level of DNA strand breakage in the modified comet experiment, as shown in Fig. 3C and 3D. This increase was observed compared to the slides solely incubated with enzyme buffers, as shown in Fig. 3B. This refers to the recognition of oxidized DNA backgrounds and the corresponding enzyme-specific DNA strand breaks on these backgrounds. After incubating with Fpg (Fig. 3C) or Endo III (Fig. 3D) endonucleases, we detected elevated levels of DNA strand breaks in the bone marrow cells obtained from BTBR mice (2.05-fold increase after Fpg incubation and 2.63-fold increase after Endo III incubation). These levels were significantly higher than those found in B6 animals. This suggests that oxidative modifications in the pyrimidine and purine bases of DNA molecules influence the DNA strand breaks observed in autistic mice. When comparing BTBR animals not treated with proanthocyanidins to those treated, it was found that the treated animals had a significantly lower level of oxidative DNA strand breaks (Fig. 3C and 3D) (P < 0.01).
3.4. Proanthocyanidins enhance the efficiency of DNA repair processes in BTBR animals
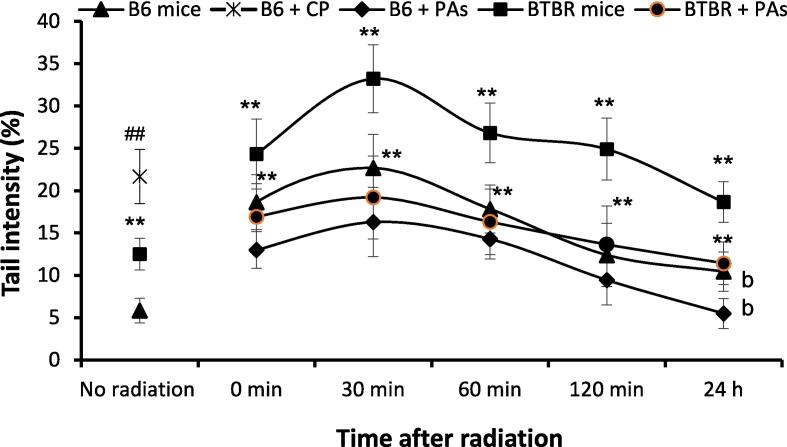
The comet test evaluated the kinetics of DNA repair in mice treated with and without proanthocyanidins. The animals were euthanized directly following gamma radiation (zero time), then at 30, 60, and 120 min or 24 h post-radiation. Fig. 4 illustrates the outline of DNA strand breakage in autistic and control mice. This pattern is characterized by an early rise of DNA strand breaks after radiation exposure, followed by a consequent decline. Autistic animals exhibited the most pronounced strand breaks, especially after a 30-min exposure to gamma radiation (5.69-fold increase). The values achieved following gamma radiation exhibited significant changes compared to sham control mice. Moreover, after gamma radiation at all-time intervals, significant differences were seen between autistic and B6 animals. Upon comparing BTBR animals to B6 animals, it was noted that B6 animals exhibited higher repair rates. More precisely, B6 animals restored approximately 45 % of DNA strand breaks induced by gamma radiation within 120 min following exposure, whereas autistic animals only managed to repair about 25 % during the same timeframe. The levels were calculated concerning their corresponding highest points at 30 min (Fig. 4). Administration of proanthocyanidins faster the repair of DNA strand breaks caused by radiation in autistic animals. Animals that were treated with proanthocyanidins exhibited significant reductions in DNA strand breaks produced by gamma radiation exposure at all-time points in both strains, demonstrating an improved ability to repair damaged DNA compared to animals that did not get proanthocyanidins therapy (P < 0.01).
Fig. 4.
Influence of proanthocyanidins (PAs) on the kinetics of damaged DNA repair efficacy in animals exposed to gamma radiation (means ± SD, N = 6). The tail intensity % measures the degree of DNA strand breaks. **P < 0.01 compared with the B6 mice and bP < 0.01 compared with the irradiated BTBR animals (Kruskal–Wallis test followed by Dunn’s multiple comparisons test). ##P < 0.01 compared to B6 mice (Mann-Whitney U test).
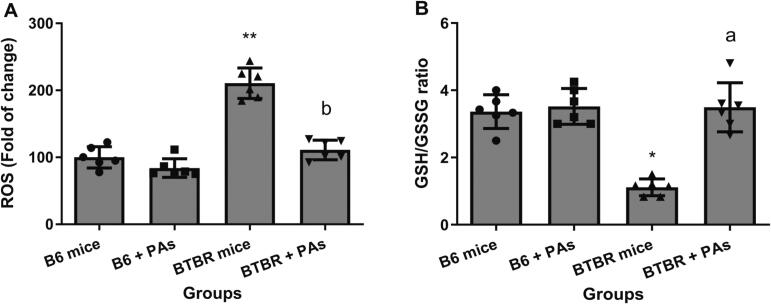
3.5. Proanthocyanidins mitigate cellular imbalance between oxidants and antioxidants in BTBR animals
The levels of redox balance were quantified by measuring the ROS production and GSH/GSSG levels. As shown in Fig. 5A, the amount of ROS produced in the B6 animals did not show any significant difference after being treated with proanthocyanidins compared to the B6 animals that were not treated. The BTBR animals exhibited a 2-fold increase in ROS generation compared to the B6 animals (P < 0.01). Nevertheless, the administration of proanthocyanidins significantly decreased the formation of ROS caused by autism, reducing it to levels significantly lower than those observed in BTBR mice who did not receive treatment (P < 0.01). In addition, there was a significant decrease in the levels of GSH in BTBR animals, together with an increase in the levels of GSSG, when compared to the levels observed in B6 animals. Subsequently, the ratio of GSH/GSSG was dramatically decreased by a factor of 3.02 in untreated BTBR animals (P < 0.05). In contrast, this ratio was significantly increased in BTBR animals treated with proanthocyanidins compared to the values in untreated BTBR animals (P < 0.01). There was no significant difference in the ratio of GSH/GSSG between the B6 animals treated with proanthocyanidins and the B6 control mice (Fig. 5B).
Fig. 5.
The impact of proanthocyanidins (PAs) on the production of reactive oxygen species (ROS) (A) and the ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG) levels in BTBR and B6 mice. ROS production was assessed by measuring the fluorescence intensity of DCF fluorescein (means ± SD, N = 6). *P < 0.05, **P < 0.01 vs. B6 control mice; aP < 0.05, bP < 0.01 vs. BTBR mice (Kruskal–Wallis test followed by Dunn’s multiple comparisons test).
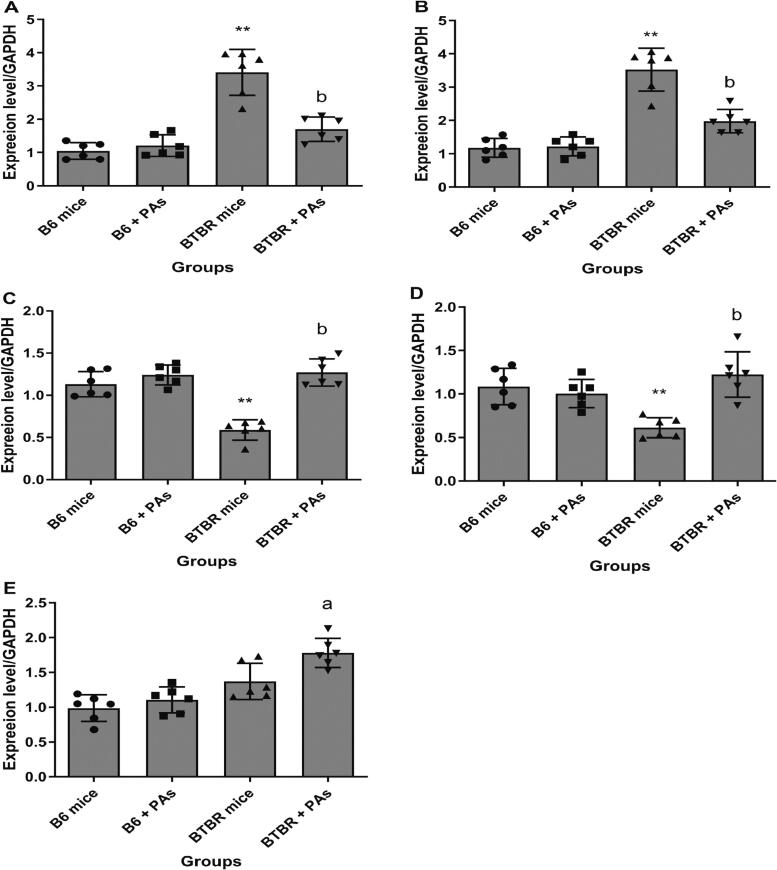
3.6. Proanthocyanidins mitigate disruptions in the expression of DNA repair genes
In B6 animals treated with proanthocyanidins, the expression levels of P53, Gadd45a, Xrcc1, Ogg1, and Parp1 showed no significant changes (Fig. 6). Furthermore, we detected no significant alteration in the P53 gene expression in BTBR mice. The expression levels of Parp1 and Gadd45a were significantly > 3-fold higher in BTBR mice compared to B6 animals (P < 0.01). Significant positive correlations were noted between elevated behavioral changes and the upregulations of Parp1 (r = 0.8234, P < 0.01) and Gadd45a (r = 0.7969, P < 0.01). Additionally, there was a significant > 1.5-fold decrease in the levels of Xrcc1 and Ogg1 (P < 0.01), with a significant correlation between elevated behavioral changes and the downregulation of these genes (P < 0.01), indicating a disruption in DNA repair pathways in BTBR animals. We observed a remarkable restoration in the expression levels of the Gadd45a, Parp1, Xrcc1, and Ogg1 genes in BTBR mice following treatment with proanthocyanidins. It was found that when proanthocyanidins were given to BTBR animals, the expression of the Xrcc1, Ogg1, and p53 genes upregulated significantly, while the expression of the Gadd45a and Parp1 genes went down significantly (P < 0.01). These findings indicate that the BTBR animals experienced enhanced DNA repair after receiving proanthocyanidins therapy.
Fig. 6.
Quantifying the expression levels of Gadd45a (A), Parp1 (B), Ogg1 (C), Xrcc1 (D), and P53 (E) repair genes in mice subjected to proanthocyanidins (PAs) treatment compared to those without treatment. The fold change in expression of repair genes is presented relative to the B6 mice used for control, which is set at a value of 1. (means ± SD, N = 6). **P < 0.01 vs. B6 control mice and aP < 0.01, bP < 0.01 vs. untreated BTBR mice (one-way ANOVA test).
4. Discussion
Autistic patients display genetic modifications, some of which are linked to DNA repair mechanisms (Markkanen et al., 2016). Therefore, patients with autism have to improve their DNA repair efficiency to maintain the integrity of their genomic DNA, in addition to managing behavioral issues. Our investigation evaluates the influence of the naturally occurring antioxidants proanthocyanidins on autism-like symptoms and the efficiency of DNA repair in the BTBR mice. In addition, the underlying mechanisms accountable for the ameliorative ability of proanthocyanidins have been studied. The present findings indicate that the repeated proanthocyanidins administration efficiently reduced autism-like behaviors in autistic animals without altering motor function. In addition, proanthocyanidins decreased the frequency of oxidative DNA strand breaks and enhanced the repair rate in the autistic animals. Autistic mice displayed elevated oxidative stress, but the BTBR mice treated with proanthocyanidins showed a significant enhancement in redox balance. Furthermore, the administration of proanthocyanidins effectively restored the impaired DNA repair genes in the BTBR mice by decreasing the expression of Gadd45a, Parp1 and increasing the expression of Xrcc1, Ogg1, and P53 genes. Importantly, repeated administrations of proanthocyanidins do not impact the assessed parameters in B6 mice, confirming the lack of toxicity of the oral administration of the extract, even at higher doses, as previously documented (Yamakoshi et al., 2002). The results indicate that proanthocyanidins have significant promise as an innovative treatment approach for patients diagnosed with autism.
Patients with autism often display repeated behaviors that can significantly limit their ability to function on a daily basis (Hodges et al., 2020). BTBR animals exhibited more disrupted repetitive behavior than B6 animals, similar to the repeated behavior reported in individuals with autism. The identified behaviors included spontaneous self-grooming, increased occurrence of burying marbles, and displaying low social interaction Autistic animals that did not receive proanthocyanidins treatment displayed an increased frequency of self-grooming and buried a more significant number of marbles than the B6 mice, which are in accordance with a previous studies (Meyza and Blanchard, 2017, Nadeem et al., 2019). Furthermore, autistic animals showed reduced social domain explorations and social proximity scores compared with B6 mice. Conversely, the administration of proanthocyanidins led to significant amelioration of these autistic behaviors. In addition, the proanthocyanidins did not affect the locomotion records as measured by the locomotor activity in the open field test, suggesting that proanthocyanidins are safe and have no effects on motor function. Previous studies have shown that proanthocyanidins have a positive impact on memory deficits and can regulate depressed and anxiety-like behaviors caused by increased oxidative stress and neuroinflammation, which aligns with our research findings (Allam et al., 2013, Jiang et al., 2017). Similarly, Jiang's study also showed that the administration of proanthocyanidins resulted in a reduction in the number of buried marbles during anxiety behavior tests. This suggests that proanthocyanidins may have an anxiolytic impact (Jiang et al., 2017). To the best of our knowledge, no prior research has been published about the influence of proanthocyanidins on repeated behaviors in autism. Thus, our study shows that proanthocyanidins have a good effect in improving behavioral abnormalities in autism. This suggests that proanthocyanidins could be an exciting treatment for managing autistic-like symptoms features.
The comet assay has been utilized in numerous investigations involving animals and human specimens to assess the spontaneous levels of DNA strand breaks in autism. The results revealed a significant increase in the incidence of endogenous DNA strand breaks in autistic specimens compared to those without the disorder (Al-Mazroua et al., 2019, Attia et al., 2020a, Attia et al., 2020b). The current study evaluated the extent of spontaneous DNA strand breaks by utilizing an alkaline comet test. B6 animals that received the positive control cyclophosphamide showed more DNA strand breakage than B6 animals. This result is consistent with the genotoxic investigations on the influences of cyclophosphamide and confirms the efficiency of the comet technique in identifying such DNA strand breakage (Attia 2012). Furthermore, the autistic mice demonstrated a considerably higher level of spontaneously occurring DNA damage than the B6 controls. Conversely, the treatment of proanthocyanidins led to a notable reduction in DNA strand break levels compared to untreated BTBR animals.
Autistic patients may have a higher incidence of DNA strand breakage, suggesting a higher vulnerability to hazardous substances and a reduced capacity for DNA repair. Numerous studies have shown that specimens isolated from autistic patients have greater susceptibility to substances that cause damage to DNA. For instance, lymphocytes obtained from children diagnosed with autism demonstrated a greater vulnerability to DNA strand breaks caused by gamma radiation and exhibited a slower rate of repairing damaged DNA in comparison to lymphocytes obtained from healthy children (Attia et al., 2020a, Attia et al., 2020b). In addition, lymphoblastoid cells derived from autistic children are more vulnerable to necrosis caused by nitrosative or oxidative stress compared to their unaffected siblings (Main et al., 2013). To evaluate the DNA repair capacity of autistic animals, the mice were exposed to gamma radiation, and a series of comet tests were performed at various time points following the radiation exposure. The results showed that the autistic animals were more susceptible to the damaging influences of gamma radiation and had a noticeably slowed recovery rate compared to the B6 mice. Furthermore, the B6 animals consistently exhibited a lower percentage of DNA damage at all assessment points, suggesting that autistic mice are more vulnerable to gamma radiation-induced strand breaks and have slower DNA repair rates.
The greater susceptibility to gamma radiation aligns with the suggested impact of specific genetic abnormalities on the DNA repair mechanisms and the aberrant expressions of genes involved in DNA repair, which are particular hallmarks of autism (Dasdemir et al., 2016, Markkanen et al., 2016). Importantly, the administration of proanthocyanidins enhanced the rate of DNA repair in autistic animals in comparison to BTBR animals that did not receive proanthocyanidins. In addition, the study found that proanthocyanidins not only accelerated the repair of DNA strand breaks caused by gamma radiation in autistic animals but also had similar benefits in the gamma-radiated B6 animals. This suggests that the impact is not influenced by the variations in mice strain. In line with our data, the same radioprotective properties were detected in mice that received proanthocyanidins prior to radiation (Huang et al., 2016, Shen et al., 2021).
Mammals possess a minimum of two protective mechanisms that combat DNA damage: antioxidant defense and DNA repair pathways. The imbalance between the antioxidants and the generation of free radicals can result in DNA damage, ultimately leading to various disorders. Oxidative stress and oxidative DNA strand breaks are linked to the development of malignancy (Attia 2010). Growing data indicates that there is a clear link between autistic symptoms and elevated levels of oxidative stress. Studies have shown that autistic animals and individuals with autism have greater levels of redox imbalance (Bjørklund et al., 2020, Liu et al., 2022a, Liu et al., 2022b).
We observed elevated baseline oxidative DNA damage levels in autistic animals by a modified comet assay that utilized the Fpg and Endo III endonucleases. Furthermore, proanthocyanidins reduced the levels of oxidative DNA strand breaks in BTBR mice compared to untreated BTBR animals. The findings of our study align with prior investigations that have indicated that proanthocyanidins have potent antioxidant activity (Arafat and Shabaan, 2019, Mahdipour et al., 2023). Previous studies have also stated that proanthocyanidins can reduce oxidative DNA strand breaks caused by DNA-damaging agents in mice (Attia et al., 2010, Bakheet et al., 2016). Likewise, our data analysis indicated that treatments with proanthocyanidins significantly reduced the increased levels of oxidative DNA strand breaks and oxidative stress, restoring them to the normal level in BTBR animals compared to BTBR mice that did not receive treatment. The data indicate that proanthocyanidins can inhibits DNA oxidation by scavenging free radicals or raising endogenous antioxidant levels, thus confirming their antioxidant properties (Nie et al., 2023). Hence, the critical pathways for lowering the elevated levels of oxidative DNA strand breaks in BTBR mice involve enhancing the antioxidant defense system and decreasing the generation of free radicals with proanthocyanidins treatment.
Several studies have confirmed the correlation between the development of autism and genetic changes. Moreover, abundant data indicates a clear relationship between increased risks of autism and gene mutation in DNA-repairing genes (Dasdemir et al., 2016, Markkanen et al., 2016). The DNA repair processes is crucial, and deficiencies in DNA repairing can results in DNA strand breaks, frequently leading to progressive cell damage and increased predisposition to cancer (Ribezzo et al., 2016); therefore, restoring the expression of genes participating in repairing damaged DNA is imperative. The current investigation examined the changes in the relative gene expressions of specific genes implicated in repairing damaged DNA. In autistic animals, Gadd45a and Parp1 expression levels were significantly higher than in B6 animals. Moreover, Xrcc1 and Ogg1 expressions were substantially decreased in BTBR mice, indicating a failure in their capacity to repair damaged DNA molecules. Nevertheless, the administration of proanthocyanidins reversed these effects. In BTBR animals, there was no noticeable change in the expression of the P53 gene. Nonetheless, BTBR animals that received proanthocyanidins displayed a notable elevation in P53 expression compared to untreated B6 and BTBR animals. These results indicate that the administration of proanthocyanidins increased DNA repair in BTBR animals by renovating the dysfunctional DNA repair machinery.
The Gadd45 family plays a role in regulating the progression of the cell cycle, apoptosis, DNA damage, and repair processes. Consequently, they participate in several biological procedures (Schmitz 2022). Numerous findings suggest that the pathology and causes of neurological diseases frequently overlap with the signaling pathways of Gadd45. For example, Gadd45 expression exhibits a substantial increase in the brains of autistic patients (Garbett et al., 2008, Huang et al., 2024). Moreover, the failure of the expression of Xrcc1 or its related protein, Ogg1, has constantly been associated with various forms of cancer and autism (Shpyleva et al., 2014, Markkanen et al., 2016, Servadio et al., 2018). Ogg1 is essential for normal brain development, and defects in Ogg1 may lead to neurodevelopmental disorders (Bhatia et al., 2022). Ogg1 facilitates the repair of the most common DNA damage induced by ROS, which has been associated with several brain function anomalies, such as autism (Zhong et al., 2024). Overexpression of Parp1 strongly influences the development of autism, and modulating Parp1 overexpression has been shown to mitigate severe autistic consequences (Dong et al., 2018). Simultaneously, suppressing Parp1 by the small chemical 3-aminobenzamide restored the cellular ability to engage in DNA repair and reduced the incidence of oxidative DNA damage (Attia et al., 2020a, Attia et al., 2020b).
BTBR mice may have inherent genetic variants that make more susceptible to oxidative damage and impaired DNA repair (Meyza and Blanchard, 2017, Kisaretova et al., 2023). These could involve genes directly related to antioxidant defenses and genes involved in DNA repair pathways. Differential expression of these genes could render BTBR mice less capable of managing oxidative DNA damage. The detected changes in the expression levels of the examined genes in our study suggest that an impaired DNA repair system could be a possible mechanism responsible for DNA strand breakage associated with autism. This results in an increase in DNA strand breaks, as identified by the comet test in our investigation. Significantly, after the administration of proanthocyanidins, the changed genes in autistic mice were notably recovered by decreasing the expressions of Parp1 and Gadd45a while enhancing the expression levels of Xrcc1 and Ogg1 genes. Our study's outcomes suggest that proanthocyanidins can defend the DNA in autistic animals by correcting redox imbalances and restoring deficient DNA repair processes.
Autism generally presents throughout the first three years of a patient's life. However, the scientists disclosed a link between disease severity and age. As patients aged, signs of autism related to communication, social interactions, and cognitive flexibility became increasingly evident (Hapṕe et al., 2016). In mice, the onset of hormonal changes and the appearance of secondary sexual characteristics associated with adolescence transpires around 42 days’ post-birth, corresponding to approximately 11.5 years in humans (Dutta and Sengupta, 2016). The animals utilized in our investigation were aged 5–7 weeks at the beginning of proanthocyanidins administration, which occurs in adolescents, enabling the observation of most behavioral alterations. As reported earlier, adolescent rodents exhibited increased novelty-evoked locomotor activities, novelty preferences, and greater exploratory relative to adult rodents (Stansfield and Kirstein 2006). The findings indicate that adolescent subjects may have a predisposition for sensation-seeking, hence increasing their likelihood of engaging in risk-taking behaviors. The current study just utilized adolescent BTBR animals, which exhibited a beneficial effect of proanthocyanidins on behavioral phenotypes and DNA repair deficiencies at this age; however, studying the effects of proanthocyanidins therapy in juvenile and adult animals might be interesting. This may bring further insights into the potential age differences in the impacts of proanthocyanidins in both mouse strains, which will be investigated more thoroughly in our future studies. Currently, healthcare providers managing autistic patients should consider the beneficial effects of proanthocyanidins, particularly throughout adolescence.
5. Conclusion
The findings suggest that the repetitive administration of proanthocyanidins effectively decreased autism-like symptoms in BTBR animals while not impacting their motor function. Proanthocyanidins decreased oxidative DNA strand breaks and improved repair efficacy in autistic animals. This activity can be explained by restoring the imbalance between oxidants and antioxidants and reversing the disturbed pathways involved in DNA repair in autistic animals. This is achieved by reducing the Gadd45a and Parp1 expressions while enhancing the Ogg1, P53, and Xrcc1 expressions. The current results suggest that proanthocyanidins have significant potential as a new therapeutic choice for people diagnosed with autism.
CRediT authorship contribution statement
Abdulelah F. Alhusain: Writing – original draft, Methodology, Conceptualization. Mohamed A. Mahmoud: Visualization, Validation, Software, Methodology. Hussain N. Alhamami: Visualization, Supervision, Formal analysis. Saad Ebrahim Alobid: Resources, Investigation, Data curation. Mushtaq A. Ansari: Visualization, Methodology. Sheikh F. Ahmad: Resources, Project administration, Data curation. Ahmed Nadeem: Validation, Funding acquisition. Saleh A. Bakheet: Resources, Investigation, Data curation. Gamaleldin I. Harisa: Project administration, Methodology, Formal analysis. Sabry M. Attia: Writing – review & editing, Writing – original draft, Methodology, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge and extend their appreciation to the Researchers Supporting Project Number (RSPD2024R748), King Saud University, Riyadh, Saudi Arabia for funding this study.
References
- Allam F., Dao A.T., Chugh G., et al. Grape powder supplementation prevents oxidative stress-induced anxiety-like behavior, memory impairment, and high blood pressure in rats. J. Nutr. 2013;143:835–842. doi: 10.3945/jn.113.174649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mazroua H.A., Alomar H.A., Ahmad S.F., et al. Assessment of DNA repair efficiency in the inbred BTBR T(+)tf/J autism spectrum disorder mouse model exposed to gamma rays and treated with JNJ7777120. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;93:189–196. doi: 10.1016/j.pnpbp.2019.04.003. [DOI] [PubMed] [Google Scholar]
- Anderson M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Arafat E.A., Shabaan D.A. The possible neuroprotective role of grape seed extract on the histopathological changes of the cerebellar cortex of rats prenatally exposed to Valproic Acid: animal model of autism. Acta Histochem. 2019;121:841–851. doi: 10.1016/j.acthis.2019.08.002. [DOI] [PubMed] [Google Scholar]
- Attia S.M. Deleterious effects of reactive metabolites. Oxid. Med. Cell. Longev. 2010;3:238–253. doi: 10.4161/oxim.3.4.13246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia S. Modulation of irinotecan-induced genomic DNA damage by theanine. Food Chem. Toxicol. 2012;50:1749–1754. doi: 10.1016/j.fct.2012.02.092. [DOI] [PubMed] [Google Scholar]
- Attia S.M. Influence of resveratrol on oxidative damage in genomic DNA and apoptosis induced by cisplatin. Mutat. Res. 2012;741:22–31. doi: 10.1016/j.mrgentox.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Attia S.M., Al-Bakheet S.A., Al-Rasheed N.M. Proanthocyanidins produce significant attenuation of doxorubicin-induced mutagenicity via suppression of oxidative stress. Oxid. Med. Cell. Longev. 2010;3:404–413. doi: 10.4161/oxim.3.6.14418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia S.M., Harisa G.I., Hassan M.H., et al. Beryllium chloride-induced oxidative DNA damage and alteration in the expression patterns of DNA repair-related genes. Mutagenesis. 2013;28:555–559. doi: 10.1093/mutage/get032. [DOI] [PubMed] [Google Scholar]
- Attia S.M., Alshahrani A.Y., Al-Hamamah M.A., et al. Dexrazoxane Averts Idarubicin-Evoked Genomic Damage by Regulating Gene Expression Profiling Associated With the DNA Damage-Signaling Pathway in BALB/c Mice. Toxicol. Sci. 2017;160:161–172. doi: 10.1093/toxsci/kfx161. [DOI] [PubMed] [Google Scholar]
- Attia S.M., Ahmad S.F., Nadeem A., et al. 3-Aminobenzamide alleviates elevated DNA damage and DNA methylation in a BTBR T(+)Itpr3(tf)/J mouse model of autism by enhancing repair gene expression. Pharmacol. Biochem. Behav. 2020;199 doi: 10.1016/j.pbb.2020.173057. [DOI] [PubMed] [Google Scholar]
- Attia S.M., Al-Hamamah M.A., Ahmad S.F., et al. Evaluation of DNA repair efficiency in autistic children by molecular cytogenetic analysis and transcriptome profiling. DNA Repair (Amst) 2020;85 doi: 10.1016/j.dnarep.2019.102750. [DOI] [PubMed] [Google Scholar]
- Bakheet S.A., Alhuraishi A.M., Al-Harbi N.O., et al. Alleviation of Aflatoxin B1-Induced Genomic Damage by Proanthocyanidins via Modulation of DNA Repair. J. Biochem. Mol. Toxicol. 2016;30:559–566. doi: 10.1002/jbt.21823. [DOI] [PubMed] [Google Scholar]
- Bhatia S., Arslan E., Rodriguez-Hernandez L.D., et al. DNA Damage and Repair and Epigenetic Modification in the Role of Oxoguanine Glycosylase 1 in Brain Development. Toxicol. Sci. 2022;187:93–111. doi: 10.1093/toxsci/kfac003. [DOI] [PubMed] [Google Scholar]
- Bjørklund G., Tinkov A.A., Hosnedlová B., et al. The role of glutathione redox imbalance in autism spectrum disorder: a review. Free Radic. Biol. Med. 2020;160:149–162. doi: 10.1016/j.freeradbiomed.2020.07.017. [DOI] [PubMed] [Google Scholar]
- Chen H., Xu J., Lv Y., et al. Proanthocyanidins exert a neuroprotective effect via ROS/JNK signaling in MPTP-induced Parkinson's disease models in vitro and in vivo. Mol. Med. Rep. 2018;18:4913–4921. doi: 10.3892/mmr.2018.9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A.R., Duthie S.J., Dobson V.L. Direct enzymic detection of endogenous oxidative base damage in human lymphocyte DNA. Carcinogenesis. 1993;14:1733–1735. doi: 10.1093/carcin/14.9.1733. [DOI] [PubMed] [Google Scholar]
- Crawley J.N., Heyer W.D., LaSalle J.M. Autism and Cancer Share Risk Genes, Pathways, and Drug Targets. Trends Genet. 2016;32:139–146. doi: 10.1016/j.tig.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi B. Autism and cancer risk. Autism Res. 2011;4:302–310. doi: 10.1002/aur.208. [DOI] [PubMed] [Google Scholar]
- Dasdemir S., Guven M., Pekkoc K.C., et al. DNA repair gene XPD Asp312Asn and XRCC4 G-1394T polymorphisms and the risk of autism spectrum disorder. Cell. Mol. Biol. (Noisy-le-Grand) 2016;62:46–50. [PubMed] [Google Scholar]
- de Giambattista C., Ventura P., Trerotoli P., et al. Sex Differences in Autism Spectrum Disorder: Focus on High Functioning Children and Adolescents. Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.539835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D., Zielke H.R., Yeh D., et al. Cellular stress and apoptosis contribute to the pathogenesis of autism spectrum disorder. Autism Res. 2018;11:1076–1090. doi: 10.1002/aur.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte G.M., de Araújo F.E.A., da Rocha J.M.C., et al. Neuroprotective Potential of Seed Extracts: Review of In Vitro and In Vivo Studies. Nutrients. 2023;15:2502. doi: 10.3390/nu15112502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Garbett K., Ebert P.J., Mitchell A., et al. Immune transcriptome alterations in the temporal cortex of subjects with autism. Neurobiol. Dis. 2008;30:303–311. doi: 10.1016/j.nbd.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges H., Fealko C., Soares N. Autism spectrum disorder: definition, epidemiology, causes, and clinical evaluation. Transl Pediatr. 2020;9:S55–S65. doi: 10.21037/tp.2019.09.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Wang J., Liu W., et al. Advances in the role of the GADD45 family in neurodevelopmental, neurodegenerative, and neuropsychiatric disorders. Front. Neurosci. 2024;18:1349409. doi: 10.3389/fnins.2024.1349409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Zhao H., Cao K., et al. Radioprotective Effect of Grape Seed Proanthocyanidins In Vitro and In Vivo. Oxid. Med. Cell. Longev. 2016;2016:5706751. doi: 10.1155/2016/5706751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein M.H., Alameen A.A., Ansari M.A., et al. Semaglutide ameliorated autism-like behaviors and DNA repair efficiency in male BTBR mice by recovering DNA repair gene expression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2024;135 doi: 10.1016/j.pnpbp.2024.111091. [DOI] [PubMed] [Google Scholar]
- Jiang X., Liu J., Lin Q., et al. Proanthocyanidin prevents lipopolysaccharide-induced depressive-like behavior in mice via neuroinflammatory pathway. Brain Res. Bull. 2017;135:40–46. doi: 10.1016/j.brainresbull.2017.09.010. [DOI] [PubMed] [Google Scholar]
- Kao H.T., Buka S.L., Kelsey K.T., et al. The correlation between rates of cancer and autism: an exploratory ecological investigation. PLoS One. 2010;5:e9372. doi: 10.1371/journal.pone.0009372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisaretova P., Tsybko A., Bondar N., et al. Molecular Abnormalities in BTBR Mice and Their Relevance to Schizophrenia and Autism Spectrum Disorders: An Overview of Transcriptomic and Proteomic Studies. Biomedicines. 2023;11 doi: 10.3390/biomedicines11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Lin J., Zhang H., et al. Oxidative Stress in Autism Spectrum Disorder-Current Progress of Mechanisms and Biomarkers. Front. Psychiatry. 2022;13 doi: 10.3389/fpsyt.2022.813304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Yin W., Meijsen J.J., et al. Cancer risk in individuals with autism spectrum disorder. Ann. Oncol. 2022;33:713–719. doi: 10.1016/j.annonc.2022.04.006. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Madabhushi R., Pan L., Tsai L.H. DNA damage and its links to neurodegeneration. Neuron. 2014;83:266–282. doi: 10.1016/j.neuron.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdipour R., Ebrahimzadeh-Bideskan A., Hosseini M., et al. The benefits of grape seed extract in neurological disorders and brain aging. Nutr. Neurosci. 2023;26:369–383. doi: 10.1080/1028415X.2022.2051954. [DOI] [PubMed] [Google Scholar]
- Main P.A., Thomas P., Esterman A., et al. Necrosis is increased in lymphoblastoid cell lines from children with autism compared with their non-autistic siblings under conditions of oxidative and nitrosative stress. Mutagenesis. 2013;28:475–484. doi: 10.1093/mutage/get025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini M., Cerny M.E.V., Cardoso N.S., et al. Grape Seed Components as Protectors of Inflammation, DNA Damage, and Cancer. Curr Nutr Rep. 2023;12:141–150. doi: 10.1007/s13668-023-00460-5. [DOI] [PubMed] [Google Scholar]
- Markkanen E., Meyer U., Dianov G.L. DNA Damage and Repair in Schizophrenia and Autism: Implications for Cancer Comorbidity and Beyond. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17060856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyza K.Z., Blanchard D.C. The BTBR mouse model of idiopathic autism - Current view on mechanisms. Neurosci. Biobehav. Rev. 2017;76:99–110. doi: 10.1016/j.neubiorev.2016.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem, A., S. F. Ahmad, N. O. Al-Harbi, et al., 2019. Increased oxidative stress in the cerebellum and peripheral immune cells leads to exaggerated autism-like repetitive behavior due to deficiency of antioxidant response in BTBR T + tf/J mice. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 89, 245-253. https://doi.org/Doi: 10.1016/j.pnpbp.2018.09.012. [DOI] [PubMed]
- Nie F., Liu L., Cui J., et al. Oligomeric Proanthocyanidins: An Updated Review of Their Natural Sources, Synthesis, and Potentials. Antioxidants (basel). 2023;12 doi: 10.3390/antiox12051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribezzo F., Shiloh Y., Schumacher B. Systemic DNA damage responses in aging and diseases. Semin. Cancer Biol. 2016;37–38:26–35. doi: 10.1016/j.semcancer.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner, B., 2011. Fundamentals of biostatistics. Boston, Brooks/Cole, Cengage Learning Boston.
- Schmitz I. Gadd45 Proteins in Immunity 2.0. Adv. Exp. Med. Biol. 2022;1360:69–86. doi: 10.1007/978-3-030-94804-7_5. [DOI] [PubMed] [Google Scholar]
- Servadio M., Manduca A., Melancia F., et al. Impaired repair of DNA damage is associated with autistic-like traits in rats prenatally exposed to valproic acid. Eur. Neuropsychopharmacol. 2018;28:85–96. doi: 10.1016/j.euroneuro.2017.11.014. [DOI] [PubMed] [Google Scholar]
- Shen H., Han J., Liu C., et al. Grape Seed Proanthocyanidins Exert a Radioprotective Effect on the Testes and Intestines Through Antioxidant Effects and Inhibition of MAPK Signal Pathways. Front Med (lausanne). 2021;8 doi: 10.3389/fmed.2021.836528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpyleva S., Ivanovsky S., de Conti A., et al. Cerebellar oxidative DNA damage and altered DNA methylation in the BTBR T+tf/J mouse model of autism and similarities with human post mortem cerebellum. PLoS One. 2014;9:e113712. doi: 10.1371/journal.pone.0113712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman J.L., Yang M., Lord C., et al. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfield K.H., Kirstein C.L. Effects of novelty on behavior in the adolescent and adult rat. Dev. Psychobiol. 2006;48:10–15. doi: 10.1002/dev.20127. [DOI] [PubMed] [Google Scholar]
- Yamakoshi J., Saito M., Kataoka S., et al. Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem. Toxicol. 2002;40:599–607. doi: 10.1016/s0278-6915(02)00006-6. [DOI] [PubMed] [Google Scholar]
- Zhong Y., Zhang X., Feng R., et al. OGG1: An emerging multifunctional therapeutic target for the treatment of diseases caused by oxidative DNA damage. Med. Res. Rev. 2024 doi: 10.1002/med.22068. [DOI] [PubMed] [Google Scholar]