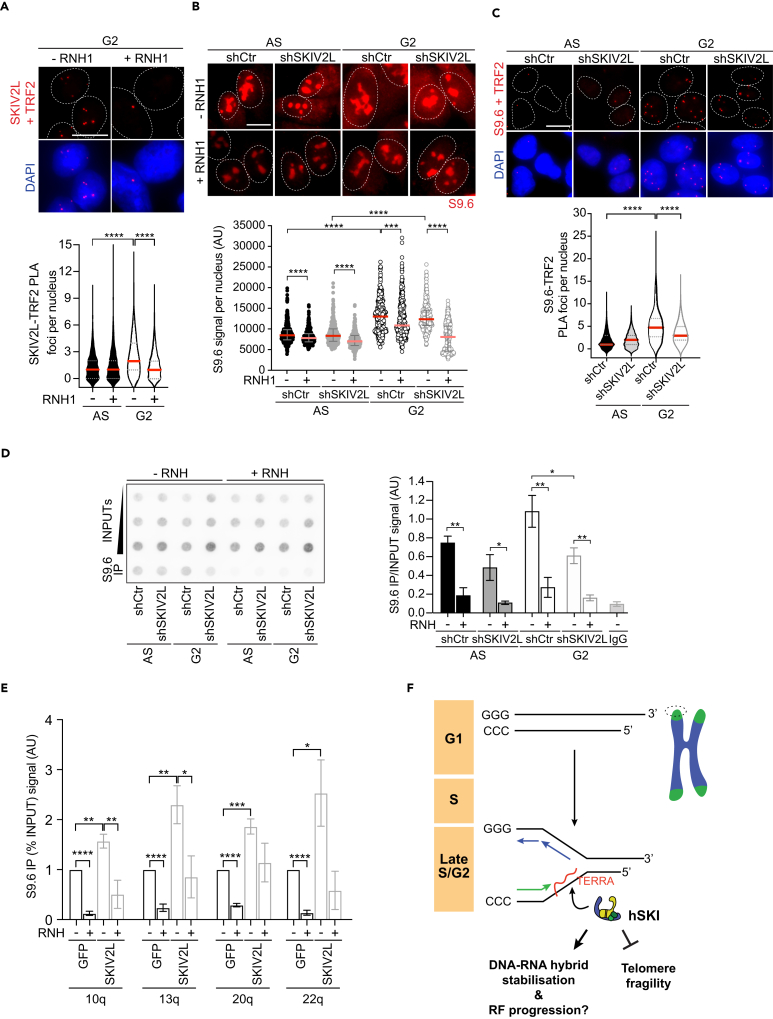
Figure 5.
SKIV2L regulates telomeric DNA-RNA hybrids in cellulo to prevent telomere fragility
(A) Proximity ligation assay (PLA) showing co-localization of SKIV2L and TRF2 in asynchronous (AS) and in G2-synchronized HeLa1.3 cells with and without RNase H1 (RNH1) overexpression (median, Q1 and Q3, at least 600 cells scored per condition, 4 independent experiments, scale bar 10 μm). Mann-Whitney U test ∗∗∗∗p < 0.0001.
(B) S9.6 IF in HeLa1.3 cells treated with RNAse III, with and without RNase H1 (RNH1) overexpression (median ± interquartile range, 360 cells scored per condition, 4 independent experiments, scale bar, 10 μm). Mann-Whitney U test ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
(C) PLA showing the co-localization of DNA-RNA hybrids (S9.6) and TRF2 in HeLa1.3 cells pre-extracted and treated with RNAse III (median, Q1 and Q3, at least 400 cells scored per condition, 2 independent experiments, scale bar 10 μm). Mann-Whitney U test ∗∗∗∗p < 0.0001.
(D) DRIP showing the levels of DNA-RNA hybrids at telomeres in AS and G2-synchronized HeLa1.3 cells, RNH, RNase H treatment (means ± SEM, n = 4).
(E) DRIP-qPCR assay of G2-synchronized HEK293 cells overexpressing GFP or SKIV2L at 10q, 13q, 20q, and 22q subtelomeric regions. RNH, RNase H treatment (means ± SEM, n = 5). Percent input values were normalized to the GFP overexpressing condition.
(F) Model proposing the function of hSKI at telomeres. Telomeric DNA-RNA hybrid accumulation in late S/G2 phase drives hSKI recruitment to telomeres to regulate physiological DNA-RNA hybrid levels, prevent telomere replication stress and ensure telomere stability. See also Figure S5.