Abstract
Background
The objective of this study was to identify antibiotic stewardship (AS) opportunities in Latin American medical–surgical intensive care units (MS-ICUs) and general wards (Gral-wards).
Methods
We conducted serial cross-sectional point prevalence surveys in MS-ICUs and Gral-wards in 41 Latin American hospitals between March 2022 and February 2023. Patients >18 years of age in the units of interest were evaluated for antimicrobial use (AU) monthly (MS-ICUs) or quarterly (Gral-wards). Antimicrobial data were collected using a standardized form by the local AS teams and submitted to the coordinating team for analysis.
Results
We evaluated AU in 5780 MS-ICU and 7726 Gral-ward patients. The hospitals’ median bed size (interquartile range) was 179 (125–330), and 52% were nonprofit. The aggregate AU prevalence was 53.5% in MS-ICUs and 25.5% in Gral-wards. Most (88%) antimicrobials were prescribed to treat infections, 7% for surgical prophylaxis and 5% for medical prophylaxis. Health care–associated infections led to 63% of MS-ICU and 38% of Gral-ward AU. Carbapenems, piperacillin-tazobactam, intravenous (IV) vancomycin, and ampicillin-sulbactam represented 50% of all AU to treat infections. A minority of IV vancomycin targeted therapy was associated with documented methicillin-resistant Staphylococcus aureus infection or therapeutic drug monitoring. In both units, 17% of antibiotics prescribed as targeted therapy represented de-escalation, while 24% and 15% in MS-ICUs and Gral-wards, respectively, represented an escalation of therapy. In Gral-wards, 32% of antibiotics were used without a microbiologic culture ordered. Half of surgical prophylaxis antibiotics were prescribed after the first 24 hours.
Conclusions
Based on this cohort, areas to improve AU in Latin American hospitals include antibiotic selection, de-escalation, duration of therapy, and dosing strategies.
Keywords: antibiotic use, antibiotic resistance, Latin America, antimicrobial stewardship, strategies
Improving antibiotic use (AU) is considered a core strategy to reduce and control antimicrobial resistance (AMR) [1, 2]. Understanding how antibiotics are prescribed is essential to designing and implementing interventions to optimize their use. Latin America has seen a rise in AMR in recent decades (eg, carbapenem nonsusceptibility among gram-negative organisms has increased from 0.3% in 2002 to 20%–50% in 2016 depending on the country according to the Latin American Network for Antimicrobial Resistance Surveillance) [3]; however, our knowledge of antibiotic stewardship (AS) priorities in the hospital setting remains limited due to scarce studies evaluating current AU patterns [4, 5].
A recent 1-day point prevalence survey (PPS) that evaluated AU in 33 Latin American hospitals from Cuba, Peru, Mexico, El Salvador, and Paraguay reported a wide range of AU prevalence (∼44% to 84% depending on the country) as well as low proportions of microbiologic studies before antibiotic initiation (as low as 20%) and of antibiotics prescribed for targeted therapy (7%–27%) [6]. Similarly, a PPS conducted in 43 intensive care units (ICUs) from Latin America reported that 30% of patients did not undergo a microbiologic culture before antibiotic initiation and 57% of antibiotics were prescribed to treat health care–associated infections (HAIs) [7]. Another study that evaluated AU in 84 ICUs from 9 Latin American countries reported high rates of AU for empiric therapy (73%–61%) and guideline compliance (59%–92%) [8]. According to a national PPS study of AU in Argentina, small hospitals had similar AU prevalence as large hospitals [9]. Limitations to published reports include data acquired at a single point in time, data from ICUs alone, and limited information on other important parameters of AU such as therapeutic drug monitoring, appropriateness of indication/dose/duration, and pharmacist involvement in antibiotic prescriptions. To address these knowledge gaps, we conducted serial PPS in medical–surgical ICUs (MS-ICUs) and general wards (Gral-wards) in 41 Latin American hospitals.
METHODS
Study Setting and Survey Methodology
Serial PPS were conducted by the local AS team in 41 hospitals from 5 countries in Latin America (Argentina, Colombia, Ecuador, Guatemala, and Panama) over 12 months (March 2022–February 2023) as part of a larger study to evaluate antibiotic stewardship program (ASP) implementation in the region. We used an adapted version of the global PPS survey. Serial 1-day PPS were performed monthly in the MS-ICUs and quarterly in the Gral-wards. All adult patients (>18 years of age) hospitalized according to the daily census in an MS-ICU or Gral-ward at 8:00 a.m. on the day of the study were evaluated for AU. Two investigators (R.E.Q. and V.F.) held virtual training webinars with local AS teams to review the data collection form and PPS methodology to ensure consistency across sites. Only patients on a systemically administered antibiotic, antimycobacterial, or antimycotic agent (J01, J02, and J04 using the World Health Organization [WHO] Anatomical Therapeutic Chemical Classification System) were evaluated for AU (ie, topical antibiotics, any antivirals, and antiparasitic agents were excluded). Specialty units (eg, psychiatric ward) and pediatric and neonatal units were excluded.
Patient Consent
The study was approved as exempt by the Johns Hopkins Medicine Institutions Review Board. Additionally, local institutional review board approvals were obtained by participating hospitals. This activity was reviewed by the Centers for Disease Control and Prevention (CDC) and was conducted consistent with applicable federal law and CDC policy (see, eg, 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.).
Data Collection and Definitions
De-identified information included basic patient characteristics and characteristics related to the antimicrobial prescription (eg, therapeutic indication, administration route, pharmacist validation, dose adjustments, and therapeutic drug monitoring). Prescriptions were categorized as treatment for community-acquired infections, treatment for HAIs, medical prophylaxis, or surgical prophylaxis. Additionally, prescriptions were adjudicated for guideline compliance based on local or international guidelines. Antibiotics for treatment of infections were further categorized as empiric or targeted (ie, based on culture results). Whether a microbiologic culture had been ordered was also recorded. Data collected by the local AS reams were stored in PROAnet, a secure regional research platform previously used for research studies by the research team, for further analysis by the Johns Hopkins University coordinating team [8, 10]. Pharmacy verification refers to a process in which pharmacists assess prescriptions for drug interactions and allergies, duplication of therapy, dosing based on organ function, etc. Anti-methicillin-resistant Staphylococcus aureus (anti-MRSA) antibiotics include daptomycin, clindamycin, linezolid, ceftaroline, and intravenous (IV) vancomycin.
Statistical Analysis
The prevalence of AU was calculated as the proportion of patients on at least 1 antimicrobial divided by the number of patients on the unit of interest (MS-ICU or Gral-ward) on PPS days, with minimum and maximum ranges. AU patterns were further analyzed using descriptive statistics focusing on antibiotics used to treat infections (“treatment antibiotics”) and those used for surgical prophylaxis. Two-sided P values <.05 were considered statistically significant. Statistical analyses were performed using Stata, version 15.1 (StataCorp LLC, College Station, TX, USA).
RESULTS
Participating Hospital Characteristics and Overall AU Prevalence
The median bed size of the 41 participating hospitals (interquartile range [IQR]) was 179 (125–330) beds, 48% were for-profit, 57% were from Argentina, 17% from Colombia, 12% from Panama, 9% from Ecuador, and 5% from Guatemala. During the 12-month study period, 41 hospitals performed a median (IQR, range) of 11 (6.5–12, 2–10) PPS in the MS-ICUs. Additionally, 34 of these hospitals also performed a median (IQR, range) of 3 (2–3, 1–12) PPS on the Gral-wards during the same study time frame.
The overall AU prevalence was 53.5% (3094/5780) in the MS-ICUs and 25.5% (1973/7726) in the Gral-wards. Most antimicrobials evaluated were antibiotics (93%), 54% of which were prescribed for empiric therapy, 34% for targeted therapy, 7% for surgical prophylaxis, and 5% for medical prophylaxis. Characteristics of patients on antimicrobials and AU prevalence by indication type are presented in Table 1.
Table 1.
Characteristics of Patients Receiving Antimicrobials, Type of Antimicrobial, and Associated Indication Included in the Analysis
| Patient Characteristics | Overall, n = 5067 |
MS-ICU, n = 3094 (61%) |
Gral-Ward, n = 1973 (39%) |
|---|---|---|---|
| Age, median (IQR), y | 58 (41–71) | 58 (41–70) | 59 (40–72) |
| Female sex, No. (%) | 2084 (41) | 1197 (39) | 887 (45) |
| Country, No. (%) | |||
| • Argentina | 3328 (66) | 1982 (64) | 1346 (63) |
| • Colombia | 788 (15) | 474 (12) | 314 (16) |
| • Ecuador | 332 (7) | 265 (9) | 67 (3) |
| • Guatemala | 224 (4) | 61 (2) | 163 (8) |
| • Panama | 395 (8) | 312 (10) | 83 (4) |
| Antimicrobials per patient, mean (range) | 1.5 (1–5) | 1.6 (1–5) | 1.3 (1–4) |
| Antimicrobial Characteristics | Overall, n = 7910 |
MS-ICU, n = 5208 (66%) |
Gral-Ward, n = 2702 (34%) |
| Type of antimicrobial, No. (%) | |||
| • Antibiotic | 7392 (93) | 4848 (94) | 2544 (94) |
| • Antifungal | 455 (58) | 321 (6) | 134 (5) |
| • Antimycobacterial | 63 (1) | 39 (1) | 24 (1) |
| Indication, No. (%) | |||
| • Medical prophylaxis | 370 (5) | 196 (4) | 174 (6) |
| • Surgical prophylaxis | 521 (7) | 287 (5) | 234 (9) |
| • Empiric treatment | 4314 (54) | 2811 (54) | 1503 (56) |
| • Targeted treatment | 2705 (34) | 1914 (37) | 791 (29) |
Serial single-day point prevalence surveys were conducted in 41 hospitals from Guatemala, Panama, Ecuador, Colombia, and Argentina during March 2022–February 2022.
Abbreviations: ICU, intensive care unit; IQR, interquartile range; Gral, general; MS, medical–surgical.
Antibiotic Use Patterns
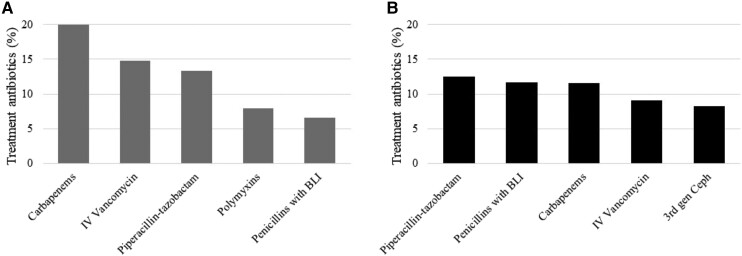
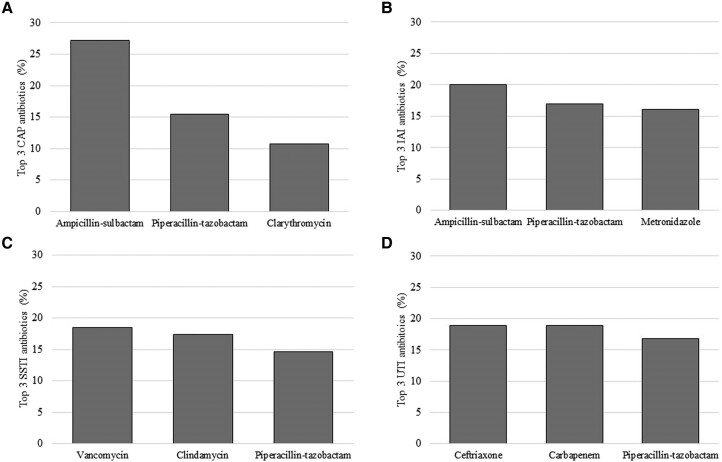
There were 4399 and 2186 antibiotics used to treat infections in the MS-ICUs and Gral-wards, respectively. The 3 most common indications for treatment antibiotics in the MS-ICUs were ventilator-associated pneumonia (n = 610, 14%), intra-abdominal infection (n = 601, 14%), and non-ventilator-associated pneumonia (n = 598, 14%), which includes community-acquired pneumonia (n = 381) and health care–associated pneumonia (n = 192). In the Gral-wards, the most common indications were skin and soft tissue infection (n = 404, 18%), urinary tract infection (n = 298, 14%), and intra-abdominal infection (n = 284, 13%) (a full list of indications by unit type is shown in Supplementary Tables 1 and 2). HAIs were responsible for 63% of MS-ICU and 38% of Gral-ward antibiotic prescriptions (see Table 2 for types of infections driving antibiotic prescriptions for treatment of infections). The 5 most common antibiotics prescribed for treatment of infections in MS-ICUs and Gral-wards are shown in Figure 1. The most common antibiotics prescribed for common community-acquired infections are summarized in Figure 2.
Table 2.
Type of Infections Driving Antibiotic Prescriptions for Treatment of Infections, by Unit Type (n = 6585)
| MS-ICU, n = 4399, No. (%) |
Gral-Ward, n = 2186, No. (%) |
|
|---|---|---|
| Community-acquired infections | 1476 (34) | 1182 (54) |
| Hospital-acquired infections | 2476 (56) | 730 (33) |
| • Surgical site infection | 399 (16) | 210 (29) |
| • Hospital-onset Clostridioides difficile infection | 49 (2) | 44 (6) |
| • Device-associated hospital-acquired infection | 897 (36) | 96 (13) |
| o Catheter-associated urinary tract infection | 89 (4) | 37 (5) |
| o Catheter-related bloodstream infection | 226 (9) | 51 (7) |
| o Ventilator-associated pneumonia | 582 (23) | 8 (1) |
| • Nondevice hospital-acquired infection | 1002 (40) | 338 (46) |
| • Other | 136 (5) | 42 (6) |
| Unknown/other | 447 (10) | 274 (12) |
Abbreviations: Gral, general; ICU, intensive care unit; MS, medical–surgical.
Figure 1.
Count of top 5 antibiotic groups used for treatment of infections in MS-ICUs (A) and Gral-wards (B). Penicillins with BLI include ampicillin-sulbactam and amoxicillin-clavulanate. Serial single-day point prevalence surveys were conducted in 41 hospitals from Guatemala, Panama, Ecuador, Colombia, and Argentina during March 2022–February 2022. Abbreviations: BLI, β-lactamase inhibitor; Gral-wards, general wards; IV, intravenous; MS-ICUs, medical–surgical intensive care units.
Figure 2.
Top 3 antibiotics prescribed for treatment of common community-acquired infections including A) community-acquired pneumoina (CAP), B) intra-abdominal infections (IAI), C) skin and soft tissue infections (SSTI), and D) urinary tract infections (UTIs). The denominator is the total number of antibiotics for each indication presented in the figure (ie, CAP treatment antibiotics, n = 551; IAI treatment antibiotics, n = 492; SSTI treatment antibiotics, n = 369; UTI treatment antibiotics, n = 190). Serial single-day point prevalence surveys were conducted in 41 hospitals from Guatemala, Panama, Ecuador, Colombia, and Argentina during March 2022–February 2022.
Gral-wards had a higher proportion of empiric AU (66% vs 61%; P ≤ .001) and a higher proportion of empiric antibiotics without a microbiologic culture ordered (32% vs 15%; P < .001) than MS-ICUs (Supplementary Tables 3 and 4). Among antibiotics used for targeted therapy, 24% and 15% of MS-ICU and Gral-ward prescriptions, respectively, represented escalation of therapy, while 17% in both units represented de-escalation (Table 3). A culture growing MRSA was present in 30% and 37% of anti-MRSA antibiotics prescribed for targeted therapy in the MS-ICUs and Gral-wards, respectively. The most common indications for which anti-MRSA antibiotics were prescribed without MRSA growing in culture included bacteremia (26%), ventilator-associated pneumonia (20%), and skin and soft tissue infection (17%). A documented infection due to an extended-spectrum β-lactamase (ESBL)–producing organism was associated with 54% of MS-ICU and 34% of Gral-ward carbapenem prescriptions.
Table 3.
Proportion of Targeted Therapy Antibiotics Maintained as Initially Prescribed, Escalated, or De-escalated by Unit Type for the Study Period
| MS-ICU, n = 1735, No. (%) |
Gral-Ward, n = 735, No. (%) |
|
|---|---|---|
| De-escalated | 301 (17) | 125 (17) |
| Escalated | 412 (24) | 107 (14) |
| Maintained | 1022 (59) | 503 (68) |
Serial single-day point prevalence surveys were conducted in 41 hospitals from Guatemala, Panama, Ecuador, Colombia, and Argentina during March 2022–February 2022.
Abbreviations: Gral, general; ICU, intensive care unit; MS, medical–surgical.
Of 5407 antibiotics adjudicated for guideline compliance, 10% were deemed noncompliant based on indication. Most patients (84% in MS-ICUs and 73% in Gral-wards) had an infectious disease (ID) consult, and in most cases their recommendations were followed (92% in MS-ICUs and 88% in Gral-wards). Antibiotic verification by pharmacy occurred in 70% of treatment antibiotics, and renal adjustment was performed in 28% of antibiotic prescriptions. Thirty-five percent of cefepime and 37% of piperacillin-tazobactam administrations in MS-ICUs were performed as prolonged infusions. Therapeutic drug monitoring was performed for 44% of MS-ICU and 31% of Gral-ward IV vancomycin used for targeted therapy, respectively, and for 22% of MS-ICU and 13% of Gral-ward aminoglycosides, respectively. Parenteral to oral switch was documented in 1.3% of MS-ICU and 4% of Gral-ward antibiotics used to treat infections.
Surgical Prophylaxis Antibiotic Use Patterns
Among surgical prophylaxis antibiotics (n = 517), first-generation cephalosporins were the most common (54%), followed by ampicillin-sulbactam (12%). Other antibiotics used for surgical prophylaxis included clindamycin (4%), fluoroquinolones (4%), metronidazole (4%), aminoglycosides (4%), vancomycin (2.5%), broad-spectrum penicillin with β-lactamase inhibitors (eg, piperacillin-tazobactam; 2%), and carbapenems (1.5%). Antibiotics were stopped within 24 hours of surgery in 49% of cases. Certain types of surgery were more likely to have postoperative prophylaxis continued beyond 24 hours from surgery (∼70% of neurosurgery, otolaryngology, and plastic surgeries) or use an alternative agent to first-generation cephalosporins or penicillin with β-lactamase inhibitors (∼40% of abdominal, plastic, and neuro surgeries each had alternative agents compared with 0%–20% in other types).
DISCUSSION
We evaluated AU patterns in MS-ICUs and Gral-wards from 41 Latin American hospitals through serial cross-sectional PPS from March 2022 to February 2023. This multicenter evaluation revealed several potential opportunities for AS intervention, including optimization of antibiotic selection for empiric and targeted therapy for common infections, de-escalation, dosing strategies, and duration.
This study was carried out in a mix of private and public medium-sized hospitals from Argentina, Colombia, Ecuador, Guatemala, and Panama. The population analyzed was mostly middle-aged individuals hospitalized in MS-ICUs and Gral-wards. Several important findings were uncovered by this study. Although we observed a higher proportion of antibiotics prescribed for targeted therapy than previous PPS conducted in the region [6], empiric use still represented a large portion of treatment antibiotics, especially among Gral-wards where ∼70% of treatment antibiotics were used as empiric therapy. Furthermore, 30% and 15% of Gral-ward and MS-ICU antibiotics, respectively, did not have an associated culture ordered; these are critical to guide subsequent antibiotic management decisions such as de-escalation of therapy, conversion of IV to an oral option, and duration of treatment. We also found a limited number of anti-MRSA antibiotics associated with a positive MRSA culture. Most antibiotics prescribed for targeted therapy in both types of units were maintained (ie, not modified from initial therapy), and ∼25% of MS-ICU antibiotics represented an escalation of therapy. These findings may suggest suboptimal selection of antibiotics for empiric use based on local pathogens and AMR prevalence and missed opportunities for de-escalation based on clinical response or microbiologic culture data. Notably, in a recent survey that explored Latin American health care workers’ perceptions and attitudes toward AS, 22% of participants had not received education on how to select antibiotics based on culture/susceptibility results, 34% had limited access to health care facility treatment guidelines, and 51% did not have access to the hospital antibiogram [11]. These data indicate the need for more effective collaboration between the microbiology laboratory and ASPs; however, important barriers may need to be addressed first including improving staffing in the lab and resources to improve efficiency in extracting microbiology data [12].
Another notable finding was that for most antibiotic prescriptions in our study there was an ID consultation, and most of the ID recommendations were accepted by the primary teams. This highlights the opportunity to strengthen education on AS principles among ID consultants and the collaboration between the AS team and ID specialists. A study from Switzerland showed that ∼40% of patients with an ID consult had at least 1 opportunity to improve AU [13]. We previously reported on the challenges related to daily AS interventions by ASPs in Latin America, mostly related to lack of protected time and insufficient personnel for AS activities (eg, in a cohort of 20 Latin American hospitals with a reported ASP, daily postprescription review with feedback was performed on a regular basis in only 45% of health care facilities) [12].
Another area for potential improvement relates to antibiotic dose and administration. Continuous or prolonged infusion of β-lactam antibiotics is recommended for critically ill patients, particularly those with gram-negative infections, as this method of administration has been associated with improved patient outcomes (reduced mortality and increase in clinical cure) [14]. In our study, a low proportion of cefepime or piperacillin-tazobactam was administered as continuous/prolonged infusion in MS-ICUs. Further research to understand the barriers to optimal antibiotic administration strategies is needed. Therapeutic drug monitoring of IV vancomycin was also infrequent in our study. Therapeutic drug monitoring of IV vancomycin is recommended due to vancomycin's narrow range for therapeutic effect and toxicity risk, especially for patients with abnormal or unstable renal function [15, 16]. Therapeutic drug monitoring is not widely available throughout Latin America due to cost, lack of access to the technology needed to measure drug levels, and lack of trained personnel to perform or interpret results [17]. This highlights the need for AS to implement strategies to minimize unnecessary IV vancomycin use and for better integration of pharmacists in AS. Barriers to better integration of pharmacists in ASPs in Latin America include lack of pharmacist expertise in AS/clinical pharmacology, lack of protected time for clinical pharmacists to participate in AS activities, and cultural norms in which antibiotic decisions have traditionally been made exclusively by physicians [11, 12].
Up to ∼40% of Gral-ward and ∼60% of MS-ICU antibiotics were prescribed for treatment of HAIs. This is in agreement with prior reports [7, 8], and it is a reminder of the long-standing need to invest in both infection prevention and control and AS programs in Latin America [18]. Similar to other studies, we also observed a high rate of surgical prophylaxis extending beyond the first 24 hours postsurgery [6, 19]. Increasing the duration of antibiotic prophylaxis is associated with an increased risk of Clostridioides difficile colitis and acute kidney injury without a reduction in surgical site infection (SSI) and is not recommended by guidelines [20–22]. AS teams should collaborate with surgeons and anesthesiologists to ensure evidence-based best practices that have shown to reduce SSIs, such as optimal timing of antibiotic administration prior to incision, are implemented [23].
There are several limitations to this study. While serial PPS provided robustness to the data, this methodology remains suboptimal in assessing some important aspects of AU, such as parenteral to oral switch (conversion rates may be higher if evaluated upon discharge), duration of therapy, and temporal variations. However, the study significantly expanded the knowledge on key areas that would benefit from improvement. While we included a large number of hospitals, there was overrepresentation from Argentina, limiting generalizability of results. The timing of the study may have biased selection of participating hospitals (eg, those more affected by external factors such as coronavirus disease 2019 surges may have been less available to participate). We did not assess the availability of antibiotics at participating hospitals, which may have impacted antibiotic choices. However, the fact that antibiotic therapy was often escalated indicates that antibiotic availability may not have influenced antibiotic choice heavily. Finally, while the study team provided training to local teams regarding PPS methodology, there may have been inaccuracies introduced during data collection that could lead to under- or overestimation of findings.
In summary, serial PPS of AU in Latin American MS-ICUs and Gral-wards between March 2022 and February 2023 identified several potential areas for AS intervention, including processes related to antibiotic selection for empiric use, antibiotic changes based on culture results, antibiotic dosing and administration, and duration of therapy. Improvements in these areas will likely require strengthening collaborations with microbiologists, pharmacists, and physicians, along with increasing resources to the ASP.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
The authors would like to thank Alejandra B. Salinas and Guadalupe Reyes-Morales for their assistance with coordination of the project.
Author contributions. V.F., S.E.C., and R.E.Q. designed the study, collected data, analyzed and interpreted the data, and wrote the manuscript. T.P. and F.L. designed the study, assisted with interpretation of the data, and critically reviewed the manuscript. W.R.A., B.A., A.B.A., M.F.B., M.C.B., M.P.B., M.L.B., I.B., A.C., X.C., S.C., A.M.C., R.C., W.C., S.M.C., P.C.C.T., G.C.C., C.Es., C.Ez., L.A.F., J.F., S.F., N.F., C.G.C., M.I.G., C.H.G.Q., J.A.G., L.G., F.G.T., S.L., D.L., P.R.L., C.G.L., A.L., I.L.L., G.M., D.M.M., M.M., F.M., C.M.P., C.M., L.G.M., Y.N., G.N., H.P., B.P., F.P., M.L.P., L.S.P., C.L.R., F.R.C., L.V.R., G.R., P.R.G., V.R., F.R., J.J.R., G.S., N.S., M.G.S., A.S., V.S., V.S., M.J.T., A.M.U., H.V., M.V., S.V.A.P., H.V.S., S.V., O.V., A.V., and E.W. contributed to data acquisition, interpretation of results, and review of the manuscript. All authors approved the final manuscript.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry.
Financial support. This study was funded by the US Centers for Disease Control and Prevention (contract #75D30121C11953 granted to V.F. and S.E.C.).
Contributor Information
Valeria Fabre, Division of Infectious Diseases, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Sara E Cosgrove, Division of Infectious Diseases, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Fernanda C Lessa, International Infection Control Branch, Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Twisha S Patel, International Infection Control Branch, Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Washington R Aleman, Hospital Alcivar, Guayaquil, Ecuador.
Bowen Aquiles, Hospital Sociedad de Lucha Contra el Cáncer, Guayaquil, Ecuador.
Ana B Arauz, Departamento de Medicina, Universidad de Panamá, Panama, Panama; Hospital Santo Tomas, Panama, Panama.
Maria F Barberis, Hospital Nacional Profesor Alejandro Posadas, El Palomar, Argentina.
Maria Del Carmen Bangher, Instituto de Cardiología de Corrientes “Juana Francisca Cabral,” Corrientes, Argentina.
Maria P Bernachea, Clínica Conciencia, Neuquén, Argentina.
Marisa L Bernan, Hospital Interzonal General de Agudos San Roque, Buenos Aires, Argentina.
Isabel Blanco, Pacifica Salud, Hospital Punta Pacifica, Panamá, Panama.
Antonio Cachafeiro, Pacifica Salud, Hospital Punta Pacifica, Panamá, Panama.
Ximena Castañeda, Clínica De La Mujer, Bogotá, Colombia; Hospital Mederi, Bogota, Colombia.
Sebastián Castillo, Hospital Del Tunal, Bogotá, Colombia.
Angel M Colque, Hospital Medico Policial Churruca Visca, Buenos Aires, Argentina.
Rosa Contreras, Hospital Dr. Marcial V. Quiroga, San Juan, Argentina.
Wanda Cornistein, Hospital Universitario Austral, Buenos Aires, Argentina.
Silvia Mabel Correa, Hospital Municipal de Trauma Dr. Federico Abete, Malvinas Argentinas, Argentina.
Paola Carolina Correal Tovar, Hospital Clínica Medicentro Familiar, Bogota, Colombia.
Gustavo Costilla Campero, Hospital Angel C. Padilla, Tucumán, Argentina.
Clara Esquivel, Hospital San Benito, Peten, Guatemala.
Cecilia Ezcurra, Hospital Alemán, Buenos Aires, Argentina.
Leandro A Falleroni, Hospital Provincial de Rosario, Rosario, Argentina.
Johana Fernandez, Hospital Dr. Guillermo Rawson, San Juan, Argentina.
Sandra Ferrari, Hospital Dr. Guillermo Rawson, San Juan, Argentina.
Natalia Frassone, Clínica Universitaria Privada Reina Fabiola, Córdoba, Argentina.
Carlos Garcia Cruz, Hospital Sociedad de Lucha Contra el Cáncer, Guayaquil, Ecuador.
Maria Isabel Garzón, Hospital Privado Universitario de Córdoba, Córdoba, Argentina.
Carlos H Gomez Quintero, Clínica De La Mujer, Bogotá, Colombia; Hospital Militar Central, Bogotá, Colombia.
José A Gonzalez, Hospital Irma de Lourdes Tzanetatos, Panama, Panama.
Lucrecia Guaymas, Clinica Privada Provincial, Buenos Aires, Argentina.
Fausto Guerrero-Toapanta, Hospital Carlos Andrade Marín, Quito, Ecuador.
Sandra Lambert, Hospital El Cruce, Buenos Aires, Argentina.
Diego Laplume, Hospital Nacional Profesor Alejandro Posadas, El Palomar, Argentina.
Paola R Lazarte, Maternidad Nuestra Señora De Las Mercedes De Tucumán, Tucumán, Argentina.
César G Lemir, Hospital San Bernardo, Salta, Argentina.
Angelica Lopez, Hospital Alcivar, Guayaquil, Ecuador.
Itzel L Lopez, Clinica Hospital San Fernando, Panama, Panama.
Guadalupe Martinez, Hospital San Bernardo, Salta, Argentina.
Diego M Maurizi, Hospital Municipal de Agudos Dr. Leonidas Lucero, Bahía Blanca, Argentina.
Mario Melgar, Hospital Roosevelt, Guatemala, Guatemala.
Florencia Mesplet, Hospital Cesar Milstein, Buenos Aires, Argentina.
Carlos Morales Pertuz, Hospital Del Tunal, Bogotá, Colombia.
Cristina Moreno, Hospital Metropolitano, Quito, Ecuador.
Luciana Gabriela Moya, Clínica Conciencia, Neuquén, Argentina.
Yanina Nuccetelli, Instituto de Diagnostico, La Plata, Argentina.
Glendys Núñez, Hospital Santo Tomas, Panama, Panama.
Hugo Paez, Hospital Simon Bolivar, Bogota, Colombia.
Belén Palacio, Sanatorio Allende Nueva Córdoba, Córdoba, Argentina.
Florencia Pellice, Hospital Dr. Marcial V. Quiroga, San Juan, Argentina.
Maria L Pereyra, Hospital Universitario Austral, Buenos Aires, Argentina.
Luz S Pirra, Hospital Privado Universitario de Córdoba, Córdoba, Argentina.
Carla Lorena Raffo, Hospital Municipal de Trauma Dr. Federico Abete, Malvinas Argentinas, Argentina.
Fanny Reino Choto, Hospital Carlos Andrade Marín, Quito, Ecuador.
Ligia Vence Reyes, The Panama Clinic, Panama, Panama.
Gerardo Ricoy, Hospital Medico Policial Churruca Visca, Buenos Aires, Argentina.
Polo Rodriguez Gonzalez, Hospital Clínica Medicentro Familiar, Bogota, Colombia.
Viviana Rodriguez, Hospital Alemán, Buenos Aires, Argentina.
Federico Romero, Sanatorio Allende Nueva Córdoba, Córdoba, Argentina.
Juan J Romero, Hospital Vozandes, Quito, Ecuador.
Graciela Sadino, Clínica Universitaria Privada Reina Fabiola, Córdoba, Argentina.
Nancy Sandoval, Hospital Roosevelt, Guatemala, Guatemala.
Mirta G Silva, Hospital Zonal General de Agudos Dr. Alberto Eurnekian, Buenos Aires, Argentina.
Astrid Smud, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina.
Virginia Soria, Hospital El Cruce, Buenos Aires, Argentina.
Vanina Stanek, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina.
Maria Jose Torralvo, Hospital Mederi, Bogota, Colombia.
Alejandra M Urueña, Maternidad Nuestra Señora De Las Mercedes De Tucumán, Tucumán, Argentina.
Hugo Videla, Instituto de Diagnostico, La Plata, Argentina.
Marisol Valle, Hospital Municipal de Agudos Dr. Leonidas Lucero, Bahía Blanca, Argentina.
Silvia Vera Amate Perez, Hospital Angel C. Padilla, Tucumán, Argentina.
Hernan Vergara-Samur, Hospital Militar Central, Bogotá, Colombia.
Silvina Villamandos, Instituto de Cardiología de Corrientes “Juana Francisca Cabral,” Corrientes, Argentina.
Olmedo Villarreal, Hospital Irma de Lourdes Tzanetatos, Panama, Panama.
Alejandra Viteri, Hospital Cesar Milstein, Buenos Aires, Argentina.
Eduardo Warley, Clinica Privada Provincial, Buenos Aires, Argentina.
Rodolfo E Quiros, Sanatorio Las Lomas, Buenos Aires, Argentina.
References
- 1. Leung E, Weil DE, Raviglione M, Nakatani H; World Health Organization World Health Day Antimicrobial Resistance Technical Working Group . The WHO policy package to combat antimicrobial resistance. Bull World Health Organ 2011; 89:390–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holmes AH, Moore LS, Sundsfjord A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016; 387:176–87. [DOI] [PubMed] [Google Scholar]
- 3.Latin American Network for Antimicrobial Resistance Surveillance. ReLAVRA data visualization dashboards. Available at: https://www.paho.org/hq/index.php?option=com_content&view=article&id=15341:relavra-visualization-1&Itemid=40388&lang=en. Accessed March 2024.
- 4. da Silva JB Jr, Espinal M, Ramon-Pardo P. Antimicrobial resistance: time for action. Rev Panam Salud Publica 2020; 44:e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fabre V, Cosgrove SE, Secaira C, et al. Antimicrobial stewardship in Latin America: past, present, and future. Antimicrob Steward Healthc Epidemiol 2022; 2:e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levy Hara G, Rojas-Cortes R, Molina Leon HF, et al. Point prevalence survey of antibiotic use in hospitals in Latin American countries. J Antimicrob Chemother 2022; 77:807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Curcio D, Ali A, Duarte A, et al. Prescription of antibiotics in intensive care units in Latin America: an observational study. J Chemother 2009; 21:527–34. [DOI] [PubMed] [Google Scholar]
- 8. Quiros RE, Bardossy AC, Angeleri P, et al. Antimicrobial stewardship programs in adult intensive care units in Latin America: implementation, assessments, and impact on outcomes. Infect Control Hosp Epidemiol 2022; 43:181–90. [DOI] [PubMed] [Google Scholar]
- 9. Peralta N, Camou BI, Leszczuk K, et al. Prevalence of hospital antibiotic use in Argentina, 2018. Infect Control Hosp Epidemiol 2019; 40:1301–4. [DOI] [PubMed] [Google Scholar]
- 10. Quirós R, APZurita J, Aleman W, et al. Impact of antimicrobial stewardship programs in Latin American adult intensive care units: PROA-LATAM project. Infect Control Hosp Epidemiol 2020; 41(S1):s520 . [Google Scholar]
- 11. Fabre V, Cosgrove SE, Lessa FC, et al. Knowledge, attitudes and perceptions of Latin American healthcare workers relating to antibiotic stewardship and antibiotic use: a cross-sectional multi-country study. Antimicrob Resist Infect Control 2024; 13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fabre V, Secaira C, Cosgrove SE, et al. Deep dive into gaps and barriers to implementation of antimicrobial stewardship programs in hospitals in Latin America. Clin Infect Dis 2023; 77(Suppl 1):S53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moulin E, Boillat-Blanco N, Zanetti G, Pluss-Suard C, de Valliere S, Senn L. Point prevalence study of antibiotic appropriateness and possibility of early discharge from hospital among patients treated with antibiotics in a Swiss University Hospital. Antimicrob Resist Infect Control 2022; 11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hong LT, Downes KJ, FakhriRavari A, et al. International consensus recommendations for the use of prolonged-infusion beta-lactam antibiotics: endorsed by the American College of Clinical Pharmacy, British Society for Antimicrobial Chemotherapy, Cystic Fibrosis Foundation, European Society of Clinical Microbiology and Infectious Diseases, Infectious Diseases Society of America, Society of Critical Care Medicine, and Society of Infectious Diseases Pharmacists: an executive summary. Pharmacotherapy 2023; 43:736–9. [DOI] [PubMed] [Google Scholar]
- 15. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2020; 77:835–64. [DOI] [PubMed] [Google Scholar]
- 16. Reuter SE, Stocker SL, Alffenaar JC, et al. Optimal practice for vancomycin therapeutic drug monitoring: position statement from the Anti-infectives Committee of the International Association of Therapeutic Drug Monitoring and Clinical Toxicology. Ther Drug Monit 2022; 44:121–32. [DOI] [PubMed] [Google Scholar]
- 17. Antunes MV, Linden R, Schaiquevich P. Therapeutic drug monitoring in developing nations: assessing the current state of affairs in South America. Expert Opin Drug Metab Toxicol 2021; 17:251–4. [DOI] [PubMed] [Google Scholar]
- 18. Cornistein W, Santonato D, Novau PA, et al. Synergy between infection control and antimicrobial stewardship programs to control carbapenem-resistant Enterobacterales. Antimicrob Steward Healthc Epidemiol 2023; 3:e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodriguez VM, Clara L, Klajn D, Colque A, Herrera MP, Angeleri P. Multicenter study of adherence to guidelines on surgical prophylaxis and the determinants of non-adherence in Argentina [in Spanish]. Rev Panam Salud Publica 2020; 44:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Branch-Elliman W, O'Brien W, Strymish J, Itani K, Wyatt C, Gupta K. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg 2019; 154:590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berrios-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg 2017; 152:784–91. [DOI] [PubMed] [Google Scholar]
- 22. World Health Organization . Global guidelines for the prevention of surgical site infection. World Health Organization; 2016. [PubMed] [Google Scholar]
- 23. Sommerstein R, Troillet N, Harbarth S, et al. Timing of cefuroxime surgical antimicrobial prophylaxis and its association with surgical site infections. JAMA Netw Open 2023; 6:e2317370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.