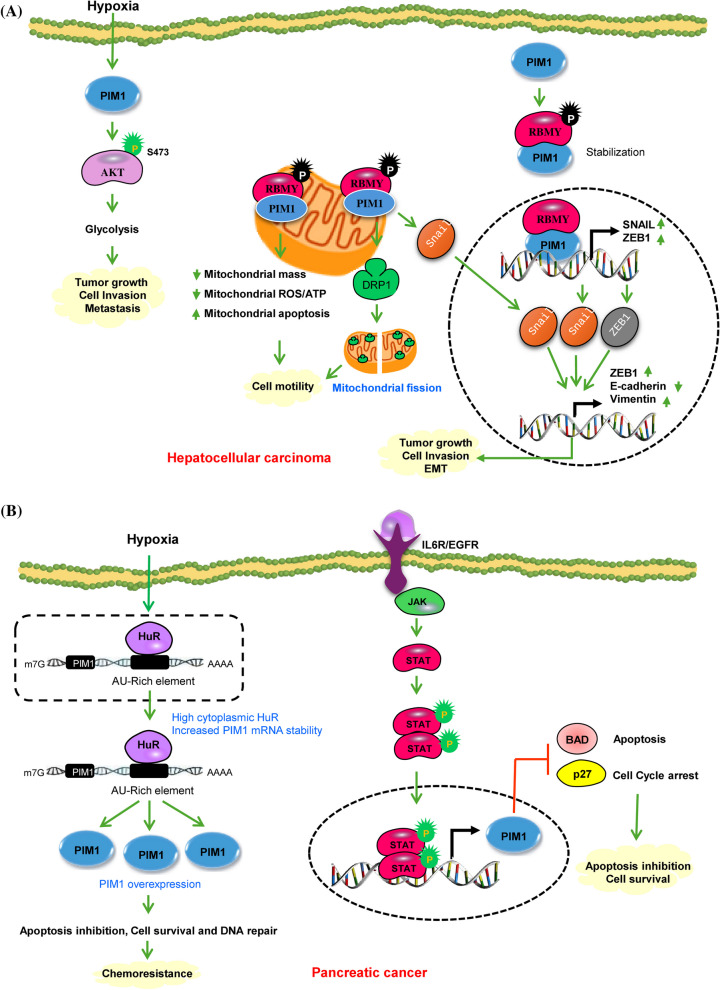
Fig. 3.
A PIM1 signaling in Hepatocellular carcinoma. PIM1 enhances AKT-driven glycolysis by phosphorylating AKT at S473, leading to the activation of its downstream targets. This process generates energy and supports the growth and advancement of HCC in the hypoxic tumor microenvironment. PIM1 phosphorylates RBMY, resulting in its cytoplasmic accumulation and activation of oncogenic functions. Upon phosphorylation PIM1 collaborates with nuclear RBMY to facilitate the transcription of Snail1 and ZEB1, and with cytoplasmic RBMY to induce the nuclear translocation of Snail1. These dual pathways synergistically boost the transcription of ZEB1 and vimentin leading to EMT and metastasis. The interaction of PIM1 with RBMY causes a reciprocal stabilization and substantial translocation of RBMY from the nuclei to the mitochondria. This PIM1-RBMY interaction prompts a shift towards mitochondrial fission, resulting in elevated ATP and ROS production for mitochondrial movement, acting as a mechanism to drive tumor metastasis. B Molecular mechanisms of PIM1 regulation in pancreatic Cancer. Under hypoxic conditions the cis-acting AU-rich elements present within a 38-base pair region of the PIM1 mRNA 3’-untranslated region mediate a regulatory interaction with the mRNA stability factor HuR. HuR, primarily expressed in the nucleus of PDAC cells, relocates to the cytoplasm in response to hypoxic stress, resulting in the stabilization and overexpression of PIM1 protein leading to enhance cell survival, DNA repair and development of chemoresistance. IL-6 stimulation triggers autophosphorylation of JAK and subsequently activates STAT3 protein. Phosphorylated STAT3, upon binding to activated JAK proteins, forms dimers that then translocate to the nucleus to control the transcription of crucial genes responsible for cell proliferation and cell cycle advancement. Green circles show activating phosphorylation events. Green and red arrows represent activating and inactivating pathways, respectively