Abstract
The biomaterial of dentin has emerged as a promising candidate for the tissue engineering of dental hard tissues. In bone tissue engineering, it may serve as either a scaffold or a reservoir of growth factors. The physical and chemical similarities between the dentin structure and bone have sparked scientific interest in using its features for the development of a new bone transplant material. Dentin, unlike hard and fragile enamel, is viscoelastic, making it a very effective bone replacement. The regeneration of pulp tissue has proven challenging due to its encasement in dentin, which lacks collateral blood flow except from the apical end of the root. Yet, the emergence of contemporary tissue engineering and the identification of dental stem cells have enabled experimentation with the regeneration of both pulp and dentin. This review will explain the different types of dentin grafts, their biocompatibility, safety, and effectiveness, along with difficulties. Additionally, the paper covers several strategies for creating autogenous dentin grafts and gives evidence-based insights into their clinical effectiveness. Overall, dentin grafts appear as a potential alternative to standard graft materials, stimulating tissue regeneration and enhancing patient outcomes in regenerative dentistry operations.
Keywords: biocompatibility, dentin grafts, osteoinductive potential, regenerative dentistry, tissue regeneration
Introduction and background
Despite major breakthroughs in dentistry, oral and dental disorders remain a vital global worry. Oral and dental treatments often use synthetic materials that possess characteristics differing from those of natural tissues, leading to their eventual failure even under optimal settings [1]. Tissue engineering is a compelling subject within the fields of regenerative medicine and dentistry. Tissue engineering, using a combination of stem cells, scaffolds, and growth factors, offers favorable prospects for the regeneration of damaged or absent tissues. Dentin is a potential biomaterial for tissue engineering, functioning as both a scaffold and a substantial source of growth factors. Dentin is a calcified connective tissue, and its properties are mostly determined by its mineralized extracellular matrix. This tissue has 50% minerals, 30% organic molecules, and 20% water [2]. However, the distribution of these components varies among several regions and types of dentin. Various clinical uses of dentin graft are shown in Table 1.
Table 1. Clinical application.
| Socket preservation |
| Periodontal defects and regeneration |
| Ridge augmentation |
| Sinus lift procedures |
| Alveolar cleft reconstruction |
| Implant site preparation |
| Root canal filling material |
| Trauma and defect repair |
In this study, we aim to explore dentin grafts, covering their history, properties, preparation, and applications. By examining current literature and recent advancements, we will assess their compatibility, effectiveness, and potential as a preferred option for dental treatments. Additionally, this review will address the challenges associated with their implantation and discuss their future potential.
Review
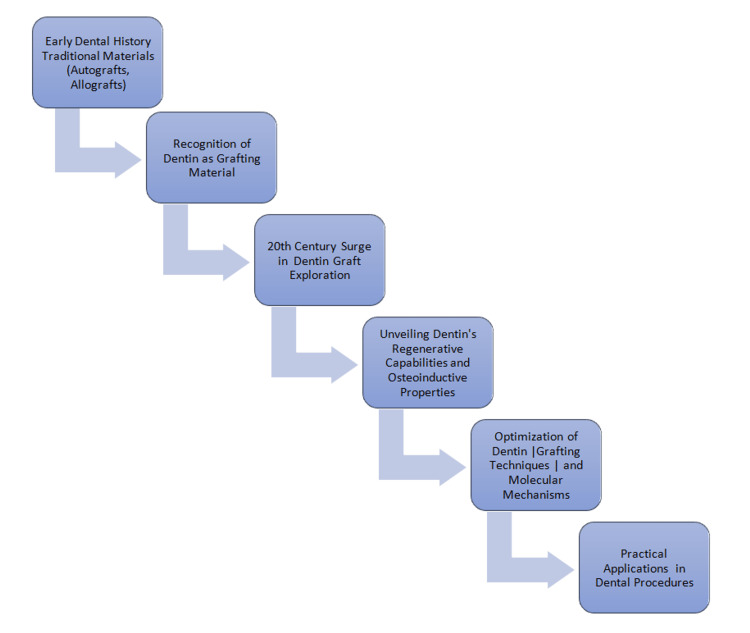
Even with significant advancements in dentistry, oral and dental illnesses continue to be a serious worldwide issue. Dental and oral treatments are often administered using artificial materials that differ from real tissues in some ways, and as a result, even under the best of circumstances, they eventually fall short. A novel concept in dentistry and regenerative medicine is tissue engineering. Tissue engineering, which combines scaffolds, growth factors, and stem cells, offers hope for the regeneration of missing or damaged tissues. Dentin is a biomaterial that shows promise for tissue engineering applications since it may function as a scaffold and a rich source of growth factors [3]. The mineralized extracellular matrix of dentin, a calcified connective tissue, is essentially responsible for its properties. Twenty percent is water, 30% is biological matter, and 50% is minerals. The distribution of these elements varies throughout dentin types and distinct parts, nevertheless. The era marked a transition from theoretical speculation to practical applications, with dental practitioners incorporating dentin grafts in diverse procedures, from periodontal treatments to implant surgeries, as shown in Figure 1.
Figure 1. Evolution of dentin as a graft material.
Image credit: Divya Suvarna Dixit
The dentin matrix’s organic composition consists of several connective tissue-specific macromolecules. This matrix also includes substances that are exclusive to mineralized tissues. The matrix is produced by odontoblasts and is a rich source of bioactive chemicals and growth factors needed for dentinogenesis. These substances are released in the event of dental caries or certain materials used in restorative procedures, and they promote dentin regeneration and repair. Ethylenediaminetetraacetic acid etchants produce dentinal matrix chemicals that exhibit strong morphogenetic activity and incite dentinogenesis in vivo. Noncollagenous and collagenous proteins, proteoglycans, glycoproteins, and lipids are all found in the dentin organic matrix. Ninety percent of the dentin extracellular matrix proteins are collagens, and type I collagen, which makes up most of the bone, is the most common kind of collagen [4]. In addition to containing several growth factors, including transforming growth factor-b, insulin-like growth factor, bone morphogenetic proteins, and certain angiogenic growth factors, dentin collagen provides a tight and crosslinked framework in which mineral crystals deposit [5]. These growth factors cause dentin disintegration and promote reparative dentinogenesis when they are released concurrently with certain events, such as the formation of cavities.
Sources of dentin graft material might vary, and recognizing the sources is vital for its effective application in different dental operations. Common sources of dentin graft material include autogenous dentin grafts, produced from the patient’s own removed teeth, commonly during regular dental operations or extractions [6]. These autogenous grafts diminish the likelihood of immunological rejection and disease transmission [7]. Another source is dentin from excised teeth, such as wisdom teeth, which repurposes native tissue and reduces the need for repeated harvesting operations [8]. Various forms of dentin grafts are presented in Table 2.
Table 2. Sources of dentin graft material.
| Source | Description | Advantages | Disadvantages |
| Autogenous dentin grafts | Derived from the patient’s own extracted teeth, minimizing the risk of immune rejection and transmission | Potential for better integration and acceptance by the host tissue | Limited availability and quantity of graft material |
| Allogenic dentin grafts | Harvested from another individual of the same species, requiring proper processing to reduce risks | Can provide a viable graft source when autogenous grafts are not feasible | Risk of immune rejection and disease transmission, necessitating thorough processing |
| Xenogenic dentin grafts | Obtained from a different species, typically porcine or bovine, with rigorous processing for safety | Potential for reduced patient morbidity as no second surgical site is required | Risk of immune response due to interspecies differences |
| Decellularized dentin matrices | Dentin matrices with removed cellular components retain structure while minimizing immunogenicity | Provides a scaffold for tissue regeneration without the risk of disease transmission | Complex processing methods are needed to remove cellular components |
| Synthetic dentin substitutes | Lab-engineered materials mimicking natural dentin properties, often based on hydroxyapatite or ceramics | Eliminates the risk of disease transmission and immune rejection | Long-term stability and integration with surrounding tissues may be a concern |
| Dentin from extracted teeth | Utilizing extracted teeth, such as wisdom teeth, as a source of graft material, repurposing natural tissue | Utilizes a readily available source without the need for an extra surgical procedure | Potential for variations in the quality and quantity of graft material |
In dental grafting, the choice between dentin grafts and traditional bone grafts is a critical decision that clinicians must make based on various factors. This comparative analysis aims to highlight the distinctions between these two approaches, shedding light on their advantages, limitations, and specific clinical considerations [9]. These factors are discussed in Table 3.
Table 3. Comparative analysis of various factors in dentin grafts and traditional bone grafts.
| Properties | Dentin graft | Traditional bone graft |
| Biological properties | Exhibit osteoinductive properties, promoting natural bone regeneration due to the presence of growth factors | Provide a scaffold for bone formation, lacking the inherent biological signaling seen in dentin |
| Osteoinductive potential | Known for their intrinsic ability to stimulate the surrounding cells and tissues for enhanced bone formation | Rely on the structural support provided by the graft, requiring a longer integration period |
| Biocompatibility | Generally well-tolerated by the host, reducing the risk of rejection | May pose a higher risk of immune response, especially with allogenic or xenogenic sources |
| Clinical applications | Widely used in various dental procedures, including socket preservation, periodontal defects, and implant placements | Applied in a range of procedures, such as ridge augmentation, sinus lifts, and complex implant cases |
| Harvesting complexity | Harvesting is typically less invasive, especially when utilizing extracted teeth or commercially available dentin products | Autogenous bone harvesting can be more invasive, requiring an additional surgical site |
| Availability and cost | Can be sourced from readily available extracted teeth, potentially reducing costs | Availability may vary, and autogenous grafts may involve higher costs and additional patient discomfort |
| Limitation | The need for additional personnel to assist with dentin graft preparation and chemical processing is a limitation of the chairside approach | Traditional bone grafting carries inherent risks of complications such as infection, graft rejection, or inadequate bone integration |
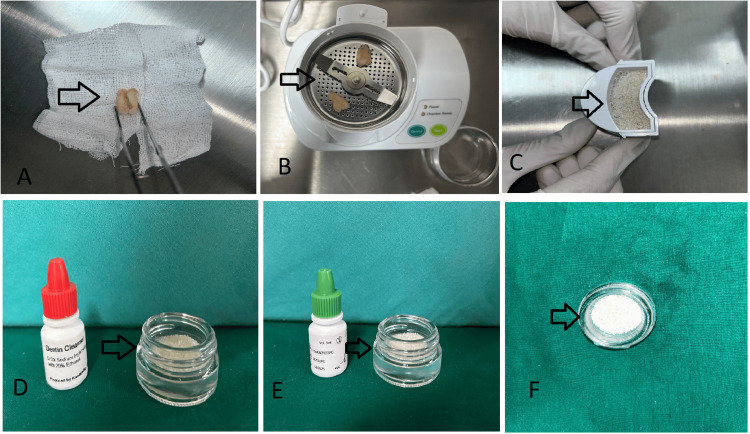
Dentin grafts have emerged as a promising avenue in regenerative dentistry, and evaluating their biocompatibility and safety is paramount for successful clinical implementation [10]. Extensive research literature provides compelling evidence supporting the favorable biocompatibility profile of dentin grafts, especially when sourced from the patient’s own teeth (autogenous). A 2021 study reports that dentin grafts are generally well-tolerated by the host, exhibiting minimal immunogenic responses and inflammatory reactions. Furthermore, it also conducts comparative analyses against traditional bone graft materials, revealing dentin’s comparable or even superior biocompatibility [11]. Long-term clinical studies have demonstrated stable outcomes and high patient satisfaction rates, affirming the safety of dentin grafts in supporting and regenerating natural bone structures [11]. In 2023, research stressed the necessity of risk mitigation techniques, including correct processing procedures and adherence to regulatory criteria, which play a key role in boosting the overall biocompatibility and safety of dentin transplants [10]. Collectively, the evidence-based insights from scientific literature underline dentin grafts as a dependable and safe alternative for numerous regenerative dental operations. With their high biocompatibility, few adverse responses, and established clinical results, dentin grafts have emerged as a viable alternative to standard graft materials in stimulating tissue regeneration and improving patient outcomes. The process of creating autogenous dentin grafts may be classified into three primary categories: mineralized, partly demineralized, and demineralized. The standard protocol entails several steps. First, the extracted tooth is cleaned and dried. Then, it is ground using a dentin grinding device to achieve a particle size of 300-1,200 μm. Next, it is treated with a solution of 0.5 molar sodium hydroxide and 20% ethanol for 12 minutes to remove any organic remains, bacteria, or toxins. Finally, the tooth is submerged in saline for three minutes, as depicted in Figure 2 [12].
Figure 2. Preparation of an autogenous dentin graft.
(A) Extracted tooth. (B) Extracted tooth in dentin grinder. (C) Dentin graft. (D) Cleaning of dentin graft. (E) Dentin graft processed. (F) Dentin graft prepared.
Image credit: Divya Suvarna Dixit
By employing just pure alcohol and sodium bicarbonate for disinfection, the mineralized dentine graft preparation method minimizes chemical treatment and maintains the tooth graft’s mineral makeup [13]. By exposing the dentine particles to diluted acids, partial demineralization occurs, removing a portion of the mineral content. This process exposes the organic dentine matrix, which contains bone-regulating proteins such as osteocalcin and osteonectin. To achieve complete demineralization, the dentine graft must be exposed to diluted acids for an extended period of time under vacuum conditions in order to remove the majority of the inorganic components, leaving behind just the organic dentine matrix [14]. The graft particles may be used as a foundation for bioengineering either by themselves or in combination with other materials, including injectable platelet-rich fibrin [15].
A search was conducted in PubMed for studies using an autogenous dentin graft prepared from an extracted tooth using a dentin grinder. The following keywords were used: “autogenous dentin graft” or dentin graft in dentistry. After examining the title and abstract, if we did not find enough information, we obtained the full text to evaluate the study’s validity for inclusion in our review. After a comprehensive screening process, a total of 25 articles were selected from an initial pool of 46 articles. The studies that met the eligibility criteria were chosen for inclusion in our review. A summary of these selected studies is presented in Table 4.
Table 4. Comparison of studies by various authors.
| Year | Author | Types of graft used | Aim of study | Parameters of study |
| 2021 | Cervera-Maillo et al. [2] | Autologous dentin graft | Using removed teeth that are ground into bacterial-free dentin fragments using a Smart Dentin Grinder and promptly transplanted into the alveolus after extraction or into bone defects, assess the effectiveness of an autologous dentin graft | Post-extraction sockets and implant gap grafted with dentin graft material, and natural healing sockets, in all cases used in the immediate post-extraction implant protocol |
| 2020 | Sánchez-Labrador et al. [11] | Autogenous dentin graft | Assessment of autogenous dentin transplant in cases with bone deficiency after excision of the lower third molar (a split-mouth clinical) | Measuring the depth of the probing at three and six months after surgery |
| 2021 | Um et al. [15] | Autogenous demineralized dentin matrix | The application of dentin graft material from other individuals — allogeneic DDM — has been considered as an alternative to auto-DDM | Steoinductivity and antigenicity of allo-DDM allogeneic dentin application for the management of maxillofacial bone defects |
| 2015 | Kim [16] | Autogenous fresh demineralized tooth graft | Analyzing the clinical utility of chairside-prepared autogenous fresh demineralized tooth grafts for alveolar bone grafting in dental implant surgery | Clinical findings, implant success rate, and histological evaluation |
| 2015 | Kim et al. [17] | Autogenous fresh demineralized tooth (block, chip, or powder) | Evaluation of chairside preparation of autogenous fresh demineralized teeth for socket preservation right after extraction in a clinical setting | Radiographic evaluation and histological evaluation of two samples |
| 2017 | El-Said et al. [18] | Autogenous fresh demineralized tooth graft | Assessing the effectiveness of chairside-prepared autogenous fresh demineralized tooth grafts for alveolar bone grafting in newly removed sockets in dental implant surgery | Clinical evaluation, radiological evaluation, and histological evaluation |
| 2019 | Fathy et al. [19] | Mineralized dentin particulate graft | An evaluation of the effects of socket preservation using mineralized dentin particle graft | Cone beam CT images were used to compare horizontal and vertical ridge diameters and bone density readings immediately post-surgical and six months after extraction |
| 2019 | Upadhyay et al. [20] | An autogenous tooth transplant made from a recently removed tooth is produced chairside | Clinical assessment of autogenous tooth graft as a new bone graft material in the treatment of Class II furcation problems | Mean reductions in horizontal probing depth and mean gains in linear bone fill |
| 2019 | Minetti et al. [21] | Extracted teeth mixed with an equal quantity of xenograft | Compare the histological results after socket preservation between dentin mixed with xenograft and dentin alone in tooth graft procedure | Three walls of post-extractive defects requiring the restoration of bone dimension and shape in the mandibular zone |
| 2019 | Wu et al. [22] | Autogenous tooth bone produced from the removed teeth by chair-side | Comparison of the efficiency of the autogenous tooth bone and xenogenic bone grafted in rapid implant placement in front teeth with a bone deficit | Clinical examination and radiographic examination of the horizontal bone change in the level of 0 mm, 3 mm, and 6mm below the implant neck and the marginal bone loss immediately, six, and 12 months after implant insertion. Questionnaire about the sentiments regarding the operation at the moment of removing the sutures |
| 2020 | Kuperschlag et al. [23] | Autogenous dentin graft | Evaluation of osseous healing after surgical excision of impacted mesioangularly or horizontally inclined third molars using the processed third molar as the graft material, followed by guided bone regeneration treatment of osseous defects distal to mandibular second molars | Clinical and radiological examinations, including panoramic radiographs and probing depths at three months and 12 months postoperatively |
| 2020 | Dwivedi and Kour [24] | Autogenous fresh mineralized tooth graft prepared at the chairside | Alveolar ridge preservation using autogenous fresh mineralized tooth graft prepared at chairside | Using a three-dimensional imaging method, the radiographic assessment of alveolar ridge preservation was carried out |
| 2021 | Yüceer-Çetiner et al. [25] | Autogenous dentin graft | Examination of the effects on bone healing processes of autogenous dentin graft and combination of autogenous dentin graft and platelet-rich fibrin injected into tooth extraction sockets | After three months, histological and immunohistochemical evaluations on the samples taken during the implant surgery. Samples obtained from each group were examined by scanning electron microscopy |
| 2022 | Gabr et al. [26] | Autogenous fresh tooth graft | Comparative analysis of autogenous fresh tooth grafts around immediate dental implants with and without platelet-rich fibrin | Peri-implant probing depth, implant stability, and radiographic evaluation of vertical and horizontal dimensional changes |
| 2022 | Shoeib et al. [27] (preprint) | Demineralized dentin particles | Contrasting the xenograft in thin buccal bone with the initial appearance of soft tissue around dental implants using dentin chips | Pink aesthetic scores after a year after implant implantation and after loading at six months. Using CBCT, the resorption of buccal and crestal bone was assessed at six and one year. Measured before loading and just after implant implantation is implant stability |
| 2022 | Gupta et al. [28] | Processed dentin particulate graft | Comparison of the efficiency of the dentin autograft with autogenous bone graft for maintenance of socket defect following removal of mandibular third teeth | Clinical and radiographic evaluation |
| 2022 | Minetti et al. [29] | Autogenous dentin particulate graft | Histological evaluation of bone after autogenous dentin particle grafting with and without a resorbable collagen membrane for alveolar ridge enhancement | Histomorphometric analysis |
| 2022 | Mazzucchi et al. [30] | Autologous dentin graft | Radiographic and periodontal assessment of post-extractive sockets | Radiographic and periodontal evaluation of post-extractive sockets |
| 2023 | Abo-El-Saad et al. [31] | Autogenous demineralized dentin graft | Comparison of alloplastic and autogenous dentin grafts combined with socket shields for the preservation of pre-implant sockets | Histological analysis, histomorphometric analysis, and radiographic analysis |
| 2023 | Sapoznikov et al. [32] | Ivory dentin graft | Ivory Dentin Graft™ in comparison with a commercial bone-derived material | Evaluating the clinical safety, tolerability, and performance |
| 2023 | Wu et al. [33] | Extrafibrillarly demineralized dentin matrix | Extrafibrillar demineralization is introduced into the construction of dentin-derived biomaterials for bone regeneration | Scaffolds for bone regeneration |
| 2023 | Feng et al. [34] | Autogenous particulate dentin | Histomorphometric and clinical efficacy of autogenous particulate dentin in alveolar ridge preservation in comparison to other grafted materials or blood clot healing | Horizontal and vertical dimensions of the extraction socket osteogenic properties degradation capacity |
In a study conducted in 2021, a comparison was made between the clinical and histological/histomorphometric outcomes of employing autogenous mineralized dentin grafts and xenograft granules for alveolar ridge preservation following tooth extraction [35]. The clinical observations, including implant stability, demonstrated similarity between both groups. However, when the histomorphometric examination was performed, it was shown that, in contrast to sockets maintained with xenograft, sockets conserved with mineralized dentin matrix showed more freshly produced bone and less leftover graft. Emerging technologies and innovations in dentin grafting include decellularized dentin matrices, which use advanced processing techniques to preserve the extracellular matrix while eliminating cellular components, improving biocompatibility, reducing immunogenicity, and enhancing scaffold properties for tissue regeneration [36]. Three-dimensional printing technology is being utilized to create customized dentin graft scaffolds with precise geometries, allowing for tailored grafts for patient-specific anatomies and promoting optimal integration and functional outcomes [37]. Biomimetic approaches focus on developing materials that closely mimic the composition and properties of natural dentin, enhancing compatibility and regenerative potential [38]. Gene therapy techniques are being explored to stimulate specific molecular pathways for accelerated dentin regeneration, offering targeted and efficient enhancement of regenerative processes and potentially reducing healing times [39]. Smart materials and drug delivery systems are being integrated into dentin grafts for controlled release of growth factors or therapeutic agents, allowing precise modulation of the graft microenvironment and promoting optimized tissue healing [40]. Nanotechnology is being incorporated into dentin grafts for improved mechanical properties and enhanced cellular interactions, increasing strength and bioactivity, and potentially expanding their applications [41]. Virtual planning tools and augmented reality are being implemented in dentin grafting procedures, improving preoperative visualization, enhancing precision, and reducing procedural risks [42]. Finally, regenerative medicine principles and stem cell therapies are being integrated to augment dentin grafting outcomes, stimulating the innate regenerative potential of tissues and leading to more robust and predictable results [43].
Conclusions
Particulate dentin grafts have to be taken into account as a substitute substance for sinus lifting, split techniques, and socket preservation. After a 24-month assessment period, one of its unique features was its high rate of resorption and noninflammatory bone repair. The open tubes found in dentin particles enabled capillaries to enter and facilitated rapid resorption. The dentin graft’s performance is at least on par with widely utilized xenogeneic or allogenic biomaterials in terms of both clinical and histological aspects. These findings underscore the potential of dentin grafts to revolutionize contemporary dental practices by offering a biocompatible and efficacious alternative to traditional biomaterials. The ability of dentin grafts to seamlessly integrate with the surrounding bone, coupled with their favorable resorption kinetics and absence of inflammatory responses, heralds a significant advancement in dental regenerative therapies. As such, the continued exploration and validation of dentin grafts through rigorous research endeavors hold the key to unlocking their full clinical utility and establishing them as a cornerstone in modern dental grafting protocols.
Disclosures
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Divya Suvarna Dixit, Bhushan P. Mundada, Nitin Bhola, Anchal Agarwal
Acquisition, analysis, or interpretation of data: Divya Suvarna Dixit, Bhushan P. Mundada, Nitin Bhola, Anchal Agarwal
Drafting of the manuscript: Divya Suvarna Dixit, Bhushan P. Mundada, Nitin Bhola, Anchal Agarwal
Critical review of the manuscript for important intellectual content: Divya Suvarna Dixit, Bhushan P. Mundada, Nitin Bhola, Anchal Agarwal
Supervision: Bhushan P. Mundada
References
- 1.Innovative concepts and recent breakthrough for engineered graft and constructs for bone regeneration: a literature systematic review. Inchingolo F, Hazballa D, Inchingolo AD, et al. Materials (Basel) 2022;15:1120. doi: 10.3390/ma15031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Autologous tooth dentin graft: a retrospective study in humans. Cervera-Maillo JM, Morales-Schwarz D, Morales-Melendez H, Mahesh L, Calvo-Guirado JL. Medicina (Kaunas) 2021;58:56. doi: 10.3390/medicina58010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pulp and dentin tissue engineering and regeneration: current progress. Huang GT. Regen Med. 2009;4:697–707. doi: 10.2217/rme.09.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murata M, Akazawa T, Mitsugi M, et al. Advances in Biomaterials Science and Biomedical Applications. Italy: IntechOpen; 2013. Autograft of dentin materials for bone regeneration. [Google Scholar]
- 5.Lee ES, Wadhwa P, Kim MK, Bo Jiang H, Um IW, Kim YM. Human Tooth and Developmental Dental Defects - Compositional and Genetic Implications. USA: IntechOpen; 2022. Organic matrix of enamel and dentin and developmental defects. [Google Scholar]
- 6.Tooth-derived bone graft material. Kim YK, Lee J, Um IW, Kim KW, Murata M, Akazawa T, Mitsugi M. J Korean Assoc Oral Maxillofac Surg. 2013;39:103–111. doi: 10.5125/jkaoms.2013.39.3.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gadhia A, Pepper T. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2024. Oral surgery, extraction of teeth. [PubMed] [Google Scholar]
- 8.Particulated wisdom teeth as an autologous bone substitute for grafting/filling material in bone defects: case report. Arabadzhiev I, Maurer P, Stevao E. J Clin Exp Dent. 2020;12:0–8. doi: 10.4317/jced.56547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bone grafts and substitutes in dentistry: a review of current trends and developments. Zhao R, Yang R, Cooper PR, Khurshid Z, Shavandi A, Ratnayake J. Molecules. 2021;26:3007. doi: 10.3390/molecules26103007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comparison of autogenous tooth materials and other bone grafts. Zhang S, Li X, Qi Y, et al. Tissue Eng Regen Med. 2021;18:327–341. doi: 10.1007/s13770-021-00333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Autogenous dentin graft in bone defects after lower third molar extraction: a split-mouth clinical trial. Sánchez-Labrador L, Martín-Ares M, Ortega-Aranegui R, López-Quiles J, Martínez-González JM. Materials (Basel) 2020;13:3090. doi: 10.3390/ma13143090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evaluation of the efficacy of mineralized dentin graft in the treatment of intraosseous defects: an experimental In vivo study. Özkahraman N, Balcıoğlu NB, Soluk Tekkesin M, Altundağ Y, Yalçın S. Medicina (Kaunas) 2022;58:103. doi: 10.3390/medicina58010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Extracted teeth: clinical waste or treasure? Melek LN, El Said MM, Abo-El-Said MM, Gad BS. J Osseointegr. 2023;15:202–209. [Google Scholar]
- 14.Platelet-rich fibrin as a bone graft material in oral and maxillofacial bone regeneration: classification and summary for better application. Liu Y, Sun X, Yu J, et al. Biomed Res Int. 2019;2019:3295756. doi: 10.1155/2019/3295756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allogeneic dentin graft: a review on its osteoinductivity and antigenicity. Um IW, Lee JK, Kim JY, Kim YM, Bakhshalian N, Jeong YK, Ku JK. Materials (Basel) 2021;14:1713. doi: 10.3390/ma14071713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Autogenous fresh demineralized tooth graft prepared at chairside for dental implant. Kim ES. Maxillofac Plast Reconstr Surg. 2015;37:8. doi: 10.1186/s40902-015-0009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Various autogenous fresh demineralized tooth forms for alveolar socket preservation in anterior tooth extraction sites: a series of 4 cases. Kim ES, Lee IK, Kang JY, Lee EY. Maxillofac Plast Reconstr Surg. 2015;37:27. doi: 10.1186/s40902-015-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evaluation of autogenous fresh demineralized tooth graft prepared at chairside for dental implant (clinical and histological study) El-Said M, Sharara A, Melek L, Khalil N. Alex Dent J. 2017;42:47–55. [Google Scholar]
- 19.Effect of using mineralized dentin particulate grafted for socket preservation. Fathy MA, Abd-ElAkher MH, Elfeky AA. https://ajdsm.journals.ekb.eg/article_108445_7ba1413d2f59bbe6f4fdb6ca56aaf228.pdf Al-Azhar J Dent Sci. 2019;22:211–215. [Google Scholar]
- 20.Treatment of furcation involvement using autogenous tooth graft with 1-year follow-up: a case series. Upadhyay P, Blaggana V, Tripathi P, Jindal M. Clin Adv Periodontics. 2019;9:4–8. doi: 10.1002/cap.10039. [DOI] [PubMed] [Google Scholar]
- 21.Autologous tooth graft: a histological comparison between dentin mixed with xenograft and dentin alone grafts in socket preservation. Minetti E, Palermo A, Savadori P, et al. https://pubmed.ncbi.nlm.nih.gov/32338473/ J Biol Regul Homeost Agents. 2019;33:189–197. [PubMed] [Google Scholar]
- 22.Immediate implant placement in anterior teeth with grafting material of autogenous tooth bone vs xenogenic bone. Wu D, Zhou L, Lin J, Chen J, Huang W, Chen Y. BMC Oral Health. 2019;19:266. doi: 10.1186/s12903-019-0970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Autogenous dentin grafting of osseous defects distal to mandibular second molars after extraction of impacted third molars. Kuperschlag A, Keršytė G, Kurtzman GM, Horowitz RA. https://pubmed.ncbi.nlm.nih.gov/32017585/ Compend Contin Educ Dent. 2020;41:76–82. [PubMed] [Google Scholar]
- 24.A neoteric procedure for alveolar ridge preservation using autogenous fresh mineralized tooth graft prepared at chair side. Dwivedi A, Kour M. J Oral Biol Craniofac Res. 2020;10:535–541. doi: 10.1016/j.jobcr.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Effect of autogenous dentin graft on new bone formation. Yüceer-Çetiner E, Özkan N, Önger ME. J Craniofac Surg. 2021;32:1354–1360. doi: 10.1097/SCS.0000000000007403. [DOI] [PubMed] [Google Scholar]
- 26.Autogenous tooth graft with platelet rich fibrin versus autogenous tooth graft only around immediate dental implant. Gabr AS, Aboelhasan MF, Ali M, Eldin M, Alashmawy MM, Elsaid MG. Int J Health Sci. 2022;1:2299–2320. [Google Scholar]
- 27.Soft tissue esthetic using pink esthetic score with autogenous dentin chips and immediate implantation versus conventional immediate implantation with xenograft in thin buccal bone: (randomized controlled clinical trial) [PREPRINT] Shoeib MA, Hamada CE, Abdelrahman AR. https://www.researchsquare.com/article/rs-2176620/v1 Res Sq. 2022 [Google Scholar]
- 28.Socket preservation using autogenous bone graft and dentin autograft after surgical removal of impacted mandibular third molar - a split-mouth study design. Gupta PS, Punde PA, Nilesh K, Patil PB, Chouradiya S, Mahalle RH. Dent Med Res. 2022;10:16–23. [Google Scholar]
- 29.Autogenous dentin particulate graft for alveolar ridge augmentation with and without use of collagen membrane: preliminary histological analysis on humans. Minetti E, Gianfreda F, Palermo A, Bollero P. Materials (Basel) 2022;15:4319. doi: 10.3390/ma15124319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Autologous dentin graft after impacted mandibular third molar extraction to prevent periodontal pocket formation-a split-mouth pilot study. Mazzucchi G, Lollobrigida M, Lamazza L, Serafini G, Di Nardo D, Testarelli L, De Biase A. Materials (Basel) 2022;15 doi: 10.3390/ma15041431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Autogenous dentin graft versus alloplastic graft combined with socket shield for pre-implant socket preservation: a split-mouth randomized clinical trial. Abo-El-Saad MM, Melek LN, Abdel Fattah HS, Ayad SS. Int J Oral Maxillofac Surg. 2023;52:1090–1096. doi: 10.1016/j.ijom.2023.01.010. [DOI] [PubMed] [Google Scholar]
- 32.A novel porcine dentin-derived bone graft material provides effective site stability for implant placement after tooth extraction: a randomized controlled clinical trial. Sapoznikov L, Haim D, Zavan B, Scortecci G, Humphrey MF. Clin Oral Investig. 2023;27:2899–2911. doi: 10.1007/s00784-023-04888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Extrafibrillarly demineralized dentin matrix for bone regeneration. Wu X, Peng W, Liu G, et al. Adv Healthc Mater. 2023;12:0. doi: 10.1002/adhm.202202611. [DOI] [PubMed] [Google Scholar]
- 34.Efficacy of autogenous particulated dentin graft for alveolar ridge preservation: a systematic review and meta-analysis of randomized controlled trials. Feng Y, Zhao R, Li J, Yuan Z, Xu X, Gong J. Medicine (Baltimore) 2023;102:0. doi: 10.1097/MD.0000000000036391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Autogenous mineralized dentin versus xenograft granules in ridge preservation for delayed implantation in post-extraction sites: a randomized controlled clinical trial with an 18 months follow-up. Santos A, Botelho J, Machado V, et al. Clin Oral Implants Res. 2021;32:905–915. doi: 10.1111/clr.13765. [DOI] [PubMed] [Google Scholar]
- 36.Decellularized extracellular matrix scaffolds: recent trends and emerging strategies in tissue engineering. Zhang X, Chen X, Hong H, Hu R, Liu J, Liu C. Bioact Mater. 2022;10:15–31. doi: 10.1016/j.bioactmat.2021.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.3D printing approach in dentistry: the future for personalized oral soft tissue regeneration. Nesic D, Schaefer BM, Sun Y, Saulacic N, Sailer I. J Clin Med. 2020;9:2238. doi: 10.3390/jcm9072238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biomimetic approaches and materials in restorative and regenerative dentistry: review article. Singer L, Fouda A, Bourauel C. BMC Oral Health. 2023;23:105. doi: 10.1186/s12903-023-02808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gene therapy for dentin regeneration with bone morphogenetic proteins. Nakashima M, Iohara K, Zheng L. Curr Gene Ther. 2006;6:551–560. doi: 10.2174/156652306778520665. [DOI] [PubMed] [Google Scholar]
- 40.The smart drug delivery system and its clinical potential. Liu D, Yang F, Xiong F, Gu N. Theranostics. 2016;6:1306–1323. doi: 10.7150/thno.14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nanotechnology-based materials as emerging trends for dental applications. Barot T, Rawtani D, Kulkarni P. Rev Adv Mater Sci. 2021;60:173–189. [Google Scholar]
- 42.Augmented reality and virtual reality in dentistry: highlights from the current research. Fahim S, Maqsood A, Das G, et al. Appl Sci. 2022;12:3719. [Google Scholar]
- 43.Role and application of stem cells in dental regeneration: a comprehensive overview. Soudi A, Yazdanian M, Ranjbar R, et al. EXCLI J. 2021;20:454–489. doi: 10.17179/excli2021-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]