Figure 2.
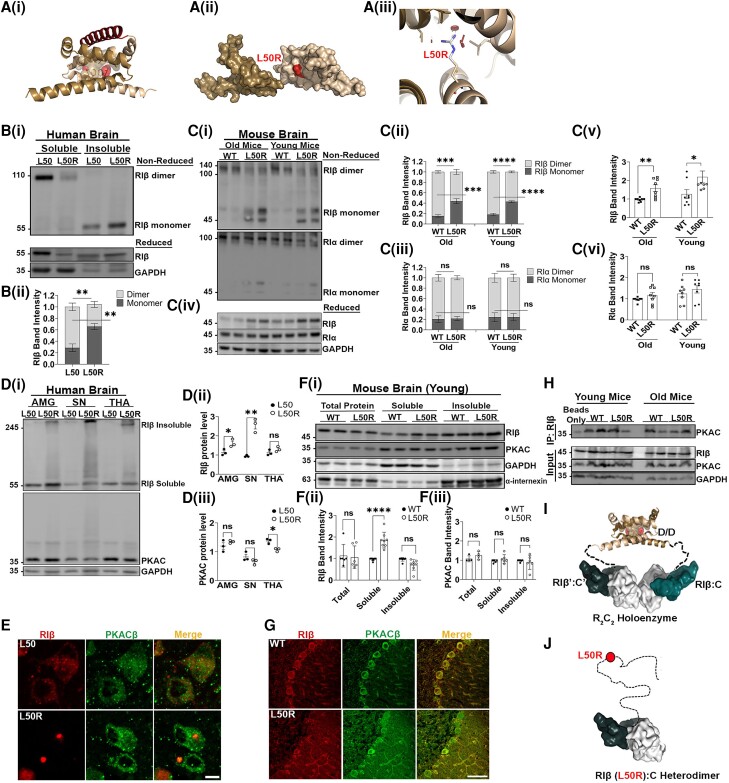
The RIβ-L50R mutant disrupts RIβ dimerization and prevents PKA holoenzyme assembly. [A(i)] Overall structure of the RIβ dimerization and docking (D/D) domain. One promoter is coloured brown and the other sand. The hydrophobic groove created by the two protomers serves as a docking site for an A-kinase anchoring protein (AKAP) peptide (red). The position of residue L50 (red spheres) in both protomers and the surrounding residues (brown spheres) are shown. [A(ii)] The position of L50R is shown as a red molecular surface between the two protomers. [A(iii)] In silico mutagenesis of the RIβ D/D domain structure (Protein Data Bank: F9K), introducing Arg instead of Leu at position 50 using PyMOL software. The model exhibits clashes with surrounding residues from the other protomer (red discs). [B(i)] Flash-frozen human cerebellum tissue lysates from a healthy individual (L50) and an individual heterozygous for the RIβ-L50R-generating mutation (L50R) were separated by sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under non-reduced (top) and reduced (bottom) conditions. In the L50 patient, RIβ is enriched in the cytosol and expressed as a homodimer, which is stabilized by inter-disulfide bonds. In the L50R patient, RIβ is found in the insoluble fraction and migrates as a monomer, confirming the breaking of dimerization. SDS-PAGE under reduced conditions compares total expression levels of RIβ between L50 and L50R in enriched fractions. GAPDH was used to determine equal loading as well as the quality of subcellular fractionation. Patient IDs (2012-95/S12/095). [B(ii)] RIβ band intensity was quantified from three independent experiments to identify the ratio between RIβ monomers to dimers. [C(i)] Tissues of soluble lysates from old (16–20 months) or young (3–4 months) of wild-type (WT) or L50R heterozygous littermate mice were separated by SDS-PAGE under non-reduced and membrane was exposed to RIβ specific antibody (top) or RIα specific antibody (bottom). [C(ii)] RIβ monomers/dimers band intensity quantifications from three independent experiments. [C(iii)] RIα monomers/dimers band intensity quantifications from three independent experiments. [C(iv)] Proteins from soluble lysates were separated by SDS-PAGE under reduced conditions to compare total expression levels of RIβ and RIa in the soluble fractions and the relative dimer formation in the non-reduced gel. GAPDH was used to determine equal loading. Band intensity for RIβ and RIa is quantified in C(v) and C(vi), respectively. Each dot represents a different mouse from at least three independent experiments. [D(i)] Flash-frozen human amygdala (AMG), substantia nigra (SN) and thalamus (THA) tissue lysates from a healthy individual (L50) and a patient carry the L50R mutation (L50R) were separated by SDS-PAGE under reduced conditions. In the L50R patient, RIβ migrates at its expected molecular weight (55 kDa) as well as in higher-order assemblies. The catalytic (C)-subunit is not detected in at higher-order assemblies and shows similar protein expression levels in both L50 and L50R patients. GAPDH was used to determine equal loading. [D(ii)] Quantification of RIβ band intensity (sum of monomer and multimer forms) from three independent experiments. Error bars represent ±standard error of the mean (SEM). Unpaired t-test. *P < 0.05, **P < 0.01; ns = non-significant comparison. [D(iii)] Quantification of C-subunit band intensity from three independent experiments. Unpaired t-test. (E) Paraffin-embedded human tissues from thalamus of L50 and L50R. The tissues were immuno-stained with anti-RIβ (red) and anti-PKACβ (PRKACB; green) antibodies. The images were acquired using confocal microscopy with ×63 magnification. Scale bar = 10 µm. [F(i)] Total cell lysates of cerebellum from WT and RIβ-L50R mutant mice at 3–4 months old were extracted and separated to soluble and insoluble fractions. Proteins were loaded on SDS-PAGE and probed with indicated antibodies. GAPDH and α-internexin were used as loading controls for soluble and insoluble fractions, respectively. [F(ii and iii)] Quantifications of band intensity of RIβ (ii) or PKAC (iii). Total proteins and soluble fractions were normalized to GAPDH and insoluble fractions were normalized to a-internexin. Each dot represents lysates from a different mouse. Error bars represent ±SEM. Unpaired t-test. **P < 0.01, ****P < 0.0001; ns; non-significant comparison. (G) IHC staining of cerebellum sagittal sections from WT and RIβ-L50R mutant young mice at 3–4 months old. The sections were labeled with anti-RIβ (red) and anti-PKACβ (green) antibodies. Images were obtained with confocal microscopy at ×63 magnification. Scale bar = 10 µm. (H) Immunoprecipitation assay using mice cerebellum lysates at both 3–4 and 16–20 months old. Lysates were immunoprecipitated with anti-RIβ antibody. Samples were loaded on SDS-PAGE and probed with anti-PKAC antibody. Lower panels: expression levels of RIβ, PKAC and GAPDH were detected in total cell lysates (Input) using specific antibodies. (I) Schematic representation of the RIβ holoenzyme structure showing how the D/D domain is extended away from the two R:C heterodimers by a flexible linker. The quaternary structure allows assemblies of RIβ:RIβ homodimerization and RIβ:C dimers of heterodimers. (J) Depiction of the RIβ-L50R mutant which retains the capacity to bind to the C-subunit but is unable to dimerize. The R-subunit is in teal, and the C-subunit is in white. PKAC = cAMP-dependent protein kinase catalytic subunit.