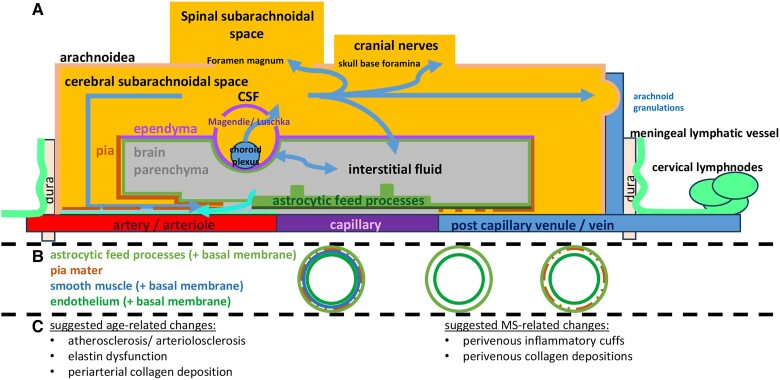
Figure 1.
The ageing perivascular compartment in multiple sclerosis. Perivascular spaces (PVS), which are implicated in brain waste removal, are involved in ageing and multiple sclerosis (MS) at different levels. CSF, produced in the choroid plexus, exchanges with brain interstitial fluid. In addition to the established CSF exit pathways along the spinal subarachnoid space, cranial nerves and arachnoid granulations, a portion of CSF flows into the brain parenchyma via the periarterial space. This flow is part of the glymphatic drainage pathway, illustrated along the arteries and through pial fenestrations. Concurrently, protein degradation products are conveyed within the muscularis of arteries, moving counter to the direction of blood flow, into the subarachnoid arteries. This process is part of the intramural peri-arterial drainage pathway, represented in blue along the artery (A). At the arterial and arteriolar level, cross-sectional views reveal that the perivascular space comprises the astrocytic end-feet processes (including their corresponding basement membrane), the pia mater (which becomes increasingly fenestrated closer to the capillary level), smooth muscle cells and the endothelium (each with their respective basement membranes). Within capillaries, the perivascular space is defined by the shared basement membranes of the astrocytic end-feet processes and the endothelium. The CSF-filled subarachnoid spaces are also evident along veins and venules, where the layers of smooth muscle cells are largely absent (B). Age-related factors, such as atherosclerosis/arteriolosclerosis, elastin dysfunction and periarterial collagen deposition, have been implicated in vascular stiffness, diminished debris transport capacity, and an increased barrier to oxygen delivery. In multiple sclerosis, perivascular changes include collagen deposition and perivenous inflammatory infiltrates that come into contact with CSF (C).