Abstract
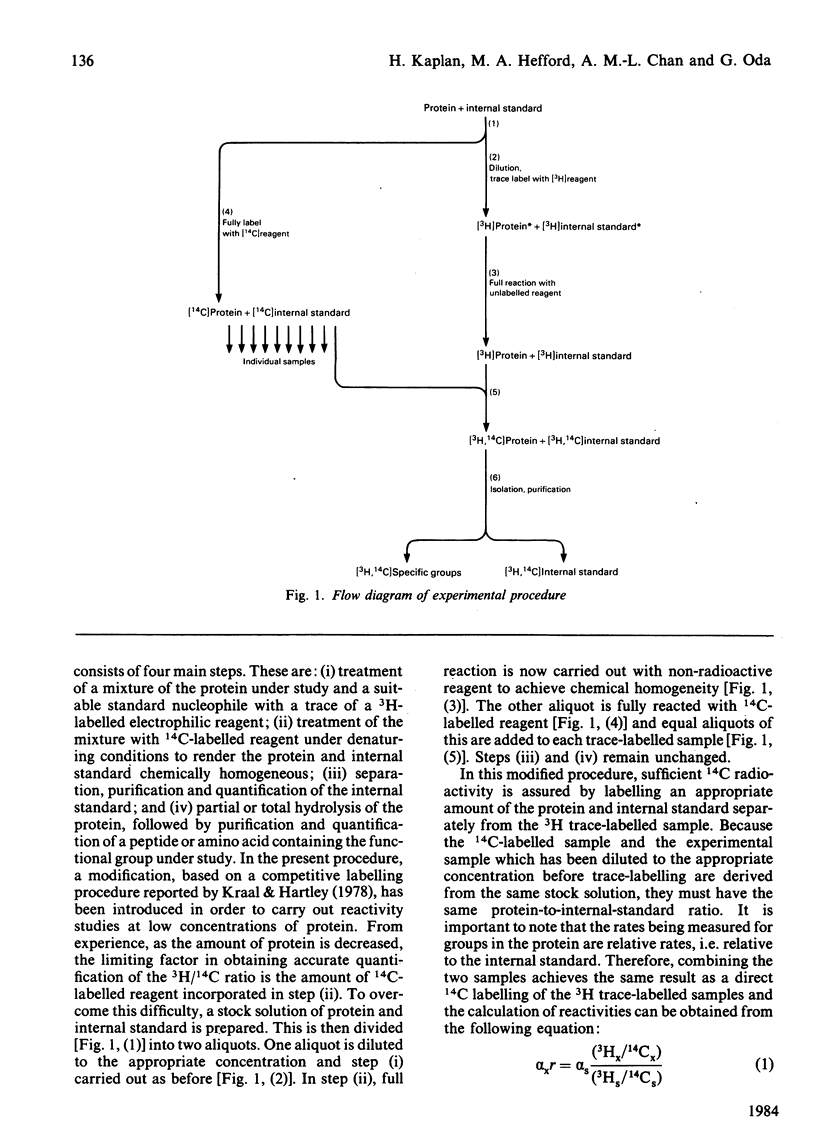
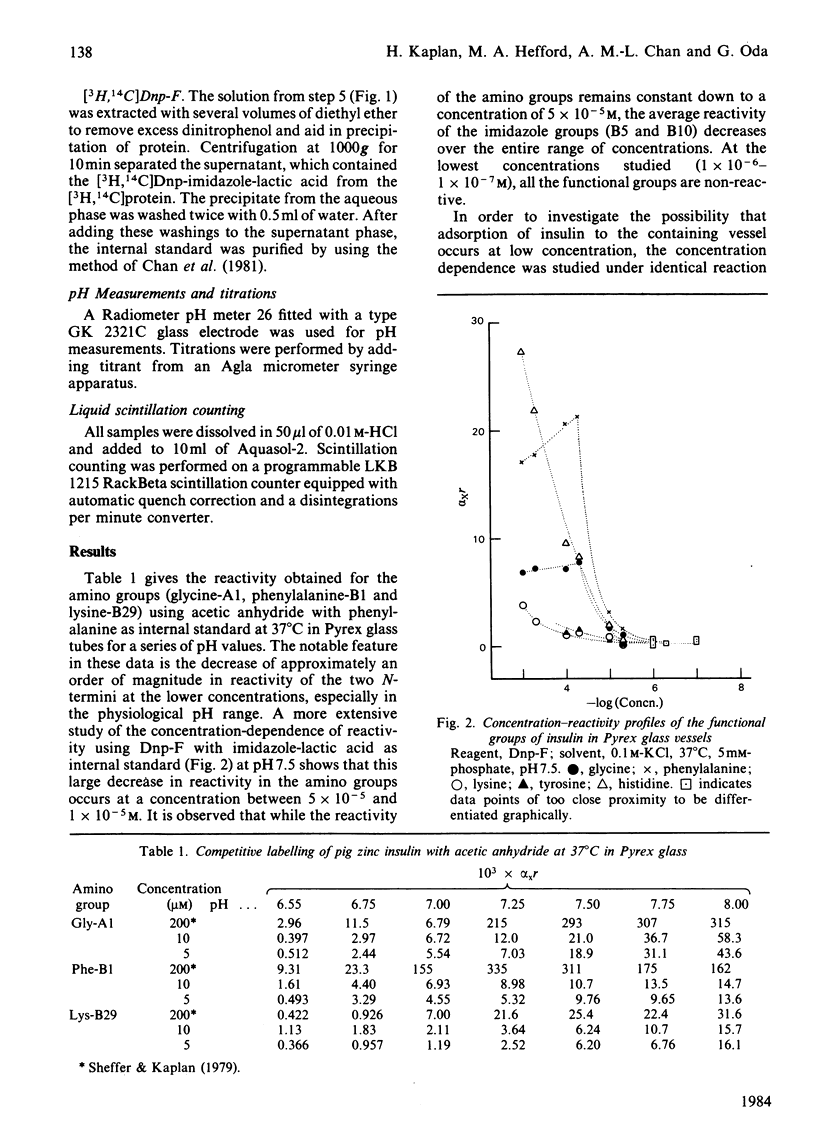
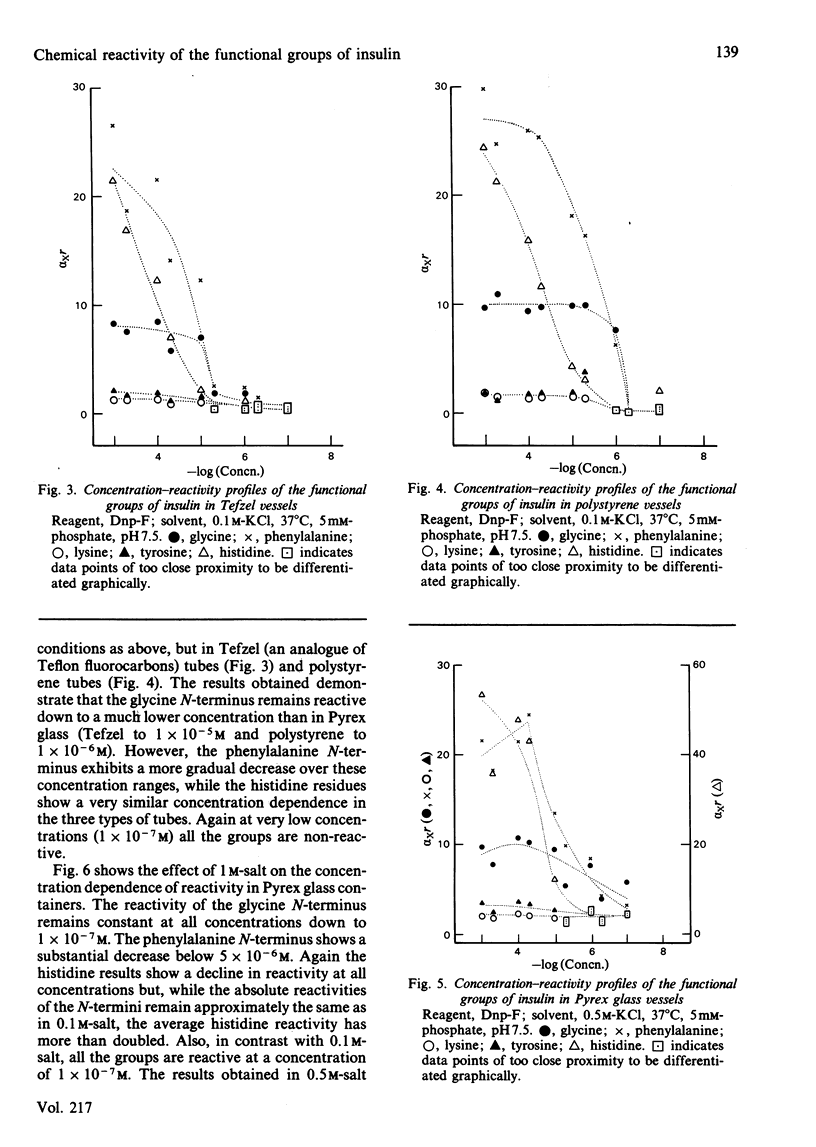
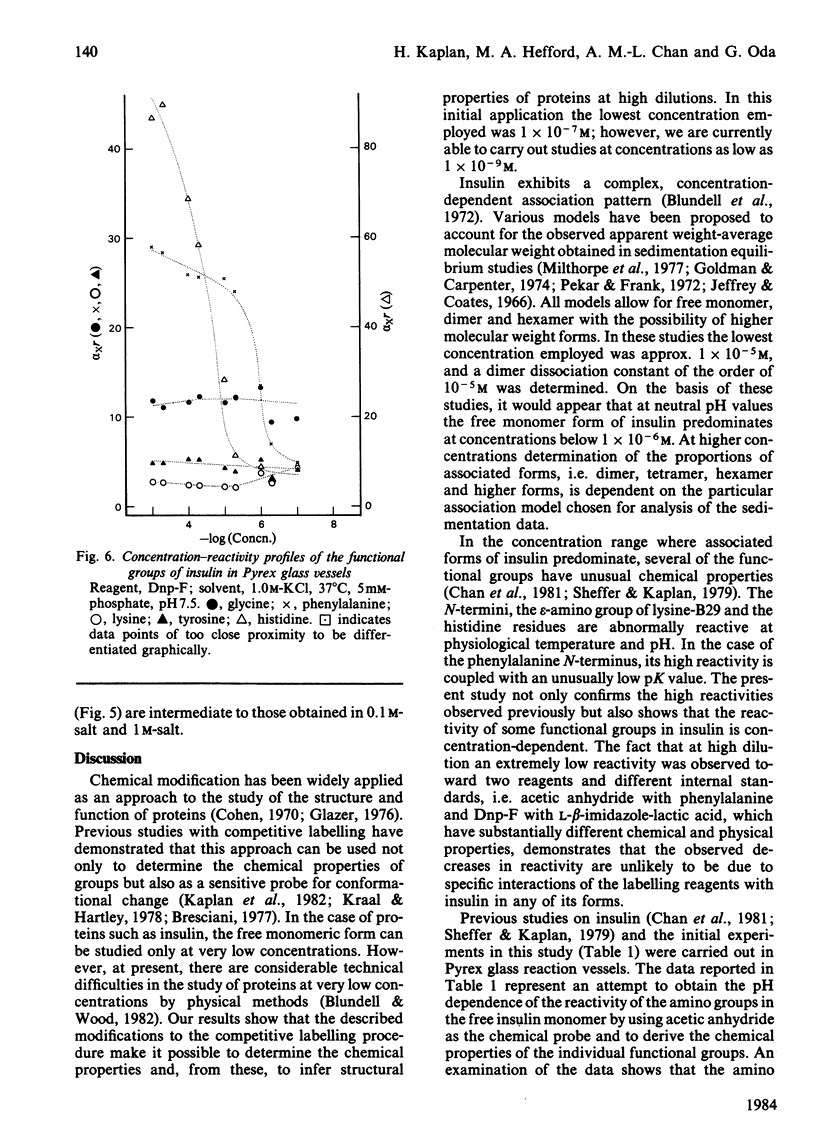
A modification to the competitive labelling procedure of Duggleby and Kaplan [(1975) Biochemistry 14, 5168-5175] was used to study the reactivity of the N-termini, lysine, histidine and tyrosine groups of insulin over the concentration range 1 X 10(-3)-1 X 10(-7)M. Reactions were carried out with acetic anhydride and 1-fluoro-2,4-dinitrobenzene in 0.1 M-KCl at 37 degrees C using Pyrex glass, Tefzel and polystyrene reaction vessels. At high concentrations all groups had either normal or enhanced reactivity but at high dilution the reactivities of all functional groups became negligible. This behaviour is attributed to the adsorption of insulin to the reaction vessels. The histidine residues show a large decrease in reactivity in all reaction vessels in the concentration range 1 X 10(-3)-1 X 10(-5)M where there are no adsorption effects and where the reactivities of all other functional groups are independent of concentration. With polystyrene, where adsorption effects become significant only below 1 X 10(-6)M, the reactivity of the phenylalanine N-terminus also shows a decrease in reactivity between 1 X 10(-5) and 1 X 10(-6)M. In 1 M-KCl insulin does not absorb to Pyrex glass and under these conditions the histidine reactivity is concentration-dependent from 1 X 10(-3) to 5 X 10(-6)M and the B1 phenylalanine alpha-amino and the B29 lysine epsilon-amino reactivities from 5 X 10(-6) to 1 X 10(-7)M, whereas the reactivities of all other groups are constant. These alterations in reactivity on dilution are attributed to disruption of dimer-dimer interactions for histidine and to monomer-monomer interactions for the phenylalanine and lysine amino groups. It is concluded that the monomeric unit of insulin has essentially the same conformation in its free and associated states.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blundell T. L., Cutfield J. F., Cutfield S. M., Dodson E. J., Dodson G. G., Hodgkin D. C., Mercola D. A., Vijayan M. Atomic positions in rhombohedral 2-zinc insulin crystals. Nature. 1971 Jun 25;231(5304):506–511. doi: 10.1038/231506a0. [DOI] [PubMed] [Google Scholar]
- Blundell T., Wood S. The conformation, flexibility, and dynamics of polypeptide hormones. Annu Rev Biochem. 1982;51:123–154. doi: 10.1146/annurev.bi.51.070182.001011. [DOI] [PubMed] [Google Scholar]
- Bresciani D. Different reactivities of free and bound amino groups in deoxy-and liganded haemoglobin. Biochem J. 1977 May 1;163(2):393–395. doi: 10.1042/bj1630393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. K., Oda G., Kaplan H. Chemical properties of the functional groups of insulin. Biochem J. 1981 Feb 1;193(2):419–425. doi: 10.1042/bj1930419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Dodson G. G., Hodgkin D. C. Transmission of conformational change in insulin. Nature. 1983 Apr 7;302(5908):500–505. doi: 10.1038/302500a0. [DOI] [PubMed] [Google Scholar]
- Cruickshank W. H., Kaplan H. A competitive labelling method for determining the ionization constants and reactivity of individual histidine residues in proteins. The histidines of -chymotrypsin. Biochem J. 1972 Dec;130(4):1125–1131. doi: 10.1042/bj1301125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank W. H., Kaplan H. Properties of the histidine residues of indole-chymotrypsin. Implications for the activation process and catalytic mechanism. Biochem J. 1975 Jun;147(3):411–416. doi: 10.1042/bj1470411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby R. G., Kaplan H. A competitive labeling method for the determination of the chemical properties of solitary functional groups in proteins. Biochemistry. 1975 Nov 18;14(23):5168–5175. doi: 10.1021/bi00694a023. [DOI] [PubMed] [Google Scholar]
- Garner M. H., Bogardt R. A., Jr, Gurd F. R. Determination of the pK values for the alpha-amino groups of human hemoglobin. J Biol Chem. 1975 Jun 25;250(12):4398–4404. [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Henkart P. The chemistry and identification of im-dinitrophenyl histidine. J Biol Chem. 1971 Apr 25;246(8):2711–2713. [PubMed] [Google Scholar]
- Holladay L. A., Ascoli M., Puett D. Conformational stability and self-association of zinc-free bovine insulin at neutral pH. Biochim Biophys Acta. 1977 Sep 27;494(1):245–254. doi: 10.1016/0005-2795(77)90152-0. [DOI] [PubMed] [Google Scholar]
- Kaplan H. Determination of the ionization constants and reactivities of the amino-termini of -chymotrypsin. J Mol Biol. 1972 Dec 14;72(1):153–162. doi: 10.1016/0022-2836(72)90076-9. [DOI] [PubMed] [Google Scholar]
- Kaplan H., Hamel P. A., Chan A. M., Oda G. Chemical properties of the N-termini of human haemoglobin. Biochem J. 1982 May 1;203(2):435–443. doi: 10.1042/bj2030435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H., Stevenson K. J., Hartley B. S. Competitive labelling, a method for determining the reactivity of individual groups in proteins. The amino groups of porcine elastase. Biochem J. 1971 Sep;124(2):289–299. doi: 10.1042/bj1240289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J. V., Rossi-Bernardi L. Interaction of hemoglobin with hydrogen ions, carbon dioxide, and organic phosphates. Physiol Rev. 1973 Oct;53(4):836–890. doi: 10.1152/physrev.1973.53.4.836. [DOI] [PubMed] [Google Scholar]
- Kraal B., Hartley B. S. Reactivity of amino groups in various complexes of the peptide chain elongation factor EF-Tu from Escherichia coli. A new method of competitive labelling using reductive methylation. J Mol Biol. 1978 Sep 25;124(3):551–564. doi: 10.1016/0022-2836(78)90187-0. [DOI] [PubMed] [Google Scholar]
- Milthorpe B. K., Nichol L. W., Jeffrey P. D. The polymerization pattern of zinc(II)-insulin at pH 7.0. Biochim Biophys Acta. 1977 Dec 20;495(2):195–202. doi: 10.1016/0005-2795(77)90376-2. [DOI] [PubMed] [Google Scholar]
- Pekar A. H., Frank B. H. Conformation of proinsulin. A comparison of insulin and proinsulin self-association at neutral pH. Biochemistry. 1972 Oct 24;11(22):4013–4016. doi: 10.1021/bi00772a001. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Biswas S. B. Conformational dynamics of insulin in solution. Circular dichroic studies. Biochemistry. 1980 Oct 28;19(22):5043–5049. doi: 10.1021/bi00563a017. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Biswas S. B. Self-association of insulin and the role of hydrophobic bonding: a thermodynamic model of insulin dimerization. Biochemistry. 1981 Jul 21;20(15):4354–4361. doi: 10.1021/bi00518a019. [DOI] [PubMed] [Google Scholar]
- Pullen R. A., Lindsay D. G., Wood S. P., Tickle I. J., Blundell T. L., Wollmer A., Krail G., Brandenburg D., Zahn H., Gliemann J. Receptor-binding region of insulin. Nature. 1976 Feb 5;259(5542):369–373. doi: 10.1038/259369a0. [DOI] [PubMed] [Google Scholar]
- Sheffer M. G., Kaplan H. Unusual chemical properties of the amino groups of insulin: implications for structure-function relationship. Can J Biochem. 1979 Jun;57(6):489–496. doi: 10.1139/o79-062. [DOI] [PubMed] [Google Scholar]
- Tatnell M. A., Jones R. H. Effects of pH and NaCl concentration on binding of covalently-linked insulin dimers to liver plasma membranes. Hoppe Seylers Z Physiol Chem. 1981 Oct;362(10):1315–1321. doi: 10.1515/bchm2.1981.362.2.1315. [DOI] [PubMed] [Google Scholar]
- Wood S. P., Blundell T. L., Wollmer A., Lazarus N. R., Neville R. W. The relation of conformation and association of insulin to receptor binding; x-ray and circular-dichroism studies on bovine and hystricomorph insulins. Eur J Biochem. 1975 Jul 15;55(3):531–542. doi: 10.1111/j.1432-1033.1975.tb02190.x. [DOI] [PubMed] [Google Scholar]


