Abstract
Malaria is a significant public health challenge in the Democratic Republic of the Congo (DR Congo), with a high prevalence and mortality rate, particularly among children under five years old. This study examines the impact of malaria in eastern DR Congo, where armed conflict and humanitarian crises have resulted in overcrowded refugee and internally displaced persons (IDP) camps, exacerbating malaria transmission. Malnutrition, limited access to healthcare, and poor living conditions makes children under the age of five particularly vulnerable. Despite attempts by organizations such as the World Health Organization and various non-governmental organizations to supply insecticide-treated bed nets and antimalarial drugs, implementation in refugee camps is impeded by war, resource constraints, and insufficient healthcare facilities. A focused elimination approach that includes integrated vector control, enhanced diagnostic access, healthcare professional training, and community engagement is critical. Surveillance and research are critical for determining malaria prevalence and resistance patterns. Effective malaria treatment in refugee camps necessitates broad strategies to protect vulnerable children and lower disease burdens.
Keywords: Antimalarial drugs, Elimination program, Children, Democratic Republic of Congo, Malaria
Highlights
-
•
Malaria is a major public and global health problem in young children in the Democratic Republic of the Congo.
-
•
The Democratic Republic of Congo has the second-highest prevalence and mortality rate of malaria globally.
-
•
Children under 5 years are vulnerable to malaria because of their immature immune systems.
1. Introduction
Malaria is considered a public health problem in the Democratic Republic of the Congo (DR Congo), which remains among the countries with a high malaria burden in the world. Globally in 2022, there were an estimated 249 million malaria cases and 608,000 malaria deaths in 85 countries [1]. According to the World Health Organization (WHO), Four African countries accounted for just over half of all malaria deaths worldwide: Nigeria (26.8 %), DR Congo (12.3 %), Uganda (5.1 %) and Mozambique (4.2 %) [1]. Eastern DR Congo, including North Kivu, South Kivu, and Ituri, has experienced long-lasting armed conflict, political instability, and recurrent humanitarian crises. All these hardships have provoked the displacement of millions of people, and therefore, the establishment of many refugees and internally displaced persons (IDP) camps [2]. The crowded and dirty conditions of these camps multiply the risk of malaria transmission. The ongoing conflict and instability further complicate efforts to manage and mitigate the spread of malaria, posing severe public health challenges and limiting access to essential medical interventions [3]. Children under five are especially vulnerable to malaria because of their immature immune systems. In the context of refugee camps, general susceptibility to the disease in the child population is enhanced by factors like malnutrition, poor health service availability, and living in overcrowded conditions. If uncomplicated malaria in young children is not timely detected and treated, it may easily progress to severe morbidity and death [4]. The vast majority of organizations that engage in the fight against malaria in DR Congo include WHO and other No-Governmental Organizations (NGOs). They distribute insecticide-treated bed nets, spray, and give antimalarial medications. However, adopting these measures into the refugee camps is a challenge [5,6]. The insecurity of the country, caused by conflict, restricts entry into the majority of the areas and poses risks for healthcare workers in the field. Also, resource constraints in terms of finances and supplies hinder the implementation of prevention and treatment interventions on an effective, comprehensive scale [7,8]. Many camps do not have proper healthcare facilities and trained personnel to manage malaria infections.
Given the high burden of malaria among children under five in refugee camps in eastern DR Congo, there is a compelling need for a targeted elimination program. Such a program should incorporate integrated vector management, including the widespread distribution, and environmental management to reduce mosquito breeding sites [9]. Also, improving diagnostic access, training healthcare workers, strengthening local health systems, ensuring medication supply, and community engagement are essential for effective malaria management [10,11]. This can be achieved through surveillance systems and research to track malaria incidence and resistance patterns [12]. The high prevalence of malaria among this age group in refugee camps underlines the necessity for targeted health interventions to protect these children and decrease the burden of disease.
2. Main text
2.1. The current state of malaria in children under 5 years in DR Congo
The DR Congo, a central African nation with an estimated population of 96 million, faces malaria as a significant public health crisis, ranking as the leading cause of disability and mortality combined [13,14]. The DR Congo has the second-highest prevalence and mortality rate of malaria globally, contributing to 12 % of the global burden [15], with 96.7 % of households in the DRC affected by malaria [16] and a prevalence rate of 13,246 cases per 100,000 population over the past two decades [17].
Children under five years old and school-age children (5–15 years) are particularly vulnerable to both symptomatic and asymptomatic malaria infections. The WHO's recommendations for malaria control, elimination, and eradication have only been partially implemented in the DR Congo. For more effective malaria control and eventual elimination, integrating all population groups into the national malaria control program is essential. Therefore, it is crucial to include schools and school-age children in the DR Congo's malaria control interventions [18].
In the DR Congo, malaria is the leading cause of illness and death among children under five, accounting for an estimated 40 % of outpatient visits and 40 % of overall mortality in this age group [19]. Malaria remains a major public health issue in Kinshasa, the capital city, which has been studied since colonial times [20]. Kinshasa is Africa's third-largest city, with a population exceeding 10 million [21]. Several factors contribute to the high burden of malaria in young children. The vast majority (97 %) of the DR Congo's population resides in areas conducive to malaria transmission, where high temperatures and stagnant water provide ideal breeding grounds for mosquitoes [22]. Access to preventive measures like insecticide-treated bed nets (ITNs) remains a challenge. Although coverage has improved, a 2017–2018 UNICEF survey revealed that only slightly over half (51 %) of children under five reported sleeping under an ITN the previous night [22].
Despite these challenges, there have been positive developments. The National Malaria Control Strategic Plan (2016–2020) targeted high-burden areas with interventions such as ITNs and improved access to diagnosis and treatment [23]. According to the President's Malaria Initiative (PMI), there has been a 34 % decrease in the mortality rate for children under five since 2010 [24].
2.2. The health implications and call for elimination program of Malaria children under 5 years old living in refugee camps
Malaria is a complex and deadly disease with an estimation of 249 million malaria cases and 608,000 malaria deaths in 85 countries. The WHO African Region carries a disproportionately high share of the global malaria burden with children less than 5 years of age accounting for about 78 % of all malaria deaths in the Region [25].
One of the factors influencing the dynamics of infectious disease transmission is human movement. This affects the rate of exposure of susceptible to infected individuals or introduce the agent to susceptible groups, or both [26]. The United Nations Refugee Agency estimated that globally, there were 89.3million forcibly displaced people [6], with almost two-thirds of them living in malaria endemic regions [27]. The high burden of malaria among the displaced communities in Africa constitutes an emerging challenge for humanitarian response [28]. Vector borne and other infectious diseases present many challenges in refugee settlements due to inequalities, limited access to healthcare services, and crowded environments which enable rapid disease transmission [29]. Children under 5 years of age are one of the population subgroups that are considered at a high risk of contracting malaria. According to data from the UNHCR, in high-transmission areas of sub-Saharan Africa malaria incidence in refugee children younger than 5 years old exceeds 1 case per child per year and accounts for 26 %–31 % of deaths [30]. When immunologically naive people who have never been infected with malaria move to areas with higher transmission intensities, their chance of contracting the disease may likely increase [27]. Refugees are more susceptible to contracting malaria infections by their lack of protective immunity, increased concentration of people in endemic settings, limited resources their poor exposed living conditions, limited distribution of ITNs, inadequate IRS, insufficient rapid clinical diagnostic tests and treatment responses. Other risk factors include outdoor night activities, inadequate body coverings or clothes, residing in unfinished houses, poor drainage and acute malnutrition among the children due to inadequate food rations [31]. Malaria elimination programmes are geared towards interrupting the transmission cycle of malaria parasite species in a defined geographical zone but can be hampered by the influx of refugees from endemic countries. This gives room for importation of malaria from these endemic zones which can contribute to secondary transmission and the spread of drug resistance and thus, threatening long-term elimination goals [32]. Interventions towards elimination should be continued and persistent efforts focused on the local realities and knowledge of factors that affect malaria distribution around the refugee camps. Hence, data and knowledge of the current prevalence of malaria among the under-fives and its associated factors in these refugee camps are needed to design appropriate and effective intervention programs [25]. The success of any elimination program implemented is largely dependent on the strength of the national health system in providing cheap, adequate and accessible services, the political will towards investing in malaria elimination strategies and other factors, including biological determinants, the environment and the social, demographic, political and economic realities of a particular country [1].
2.3. The preventions strategies and recommendations to fight malaria in children under 5 years old living in refugee camps in eastern Democratic Republic of Congo
WHO recommends the use of insecticide-treated mosquito nets (ITNs) specifically the long-lasting insecticide nets (LLINs) as an intervention in protecting populations at risk of malaria, including in areas where the risk of reintroduction remains [33]. ITNs are the only vector control widely implemented in DR Congo for the prevention of malaria, and with emphasis on children under five and pregnant women. From 2010 to 2018, the parameters of household ITN ownership, ITN use among children under five years of age and pregnant women increased from 30 %, 38 % and 43 %, to 44 %, 51 %, and 52 %, respectively [34].
WHO recommends indoor residual spraying IRS using a product prequalified by WHO for the prevention and control of malaria in children and adults living in areas with ongoing malaria transmission [35]. The Tenke Fungurume Mining (TFM) vector control component includes indoor residual spraying (IRS) of insecticides twice a year on interior wall surfaces and eave areas, initial and periodic mass redistribution of LLINs, and selective larva monitoring and control using environmental management practices and/or larvicidal agents as appropriate [36]. A minimum of three doses of IPTp (IPTp3+) with universal coverage of at least 80 % of pregnant women is recommended by WHO [37].
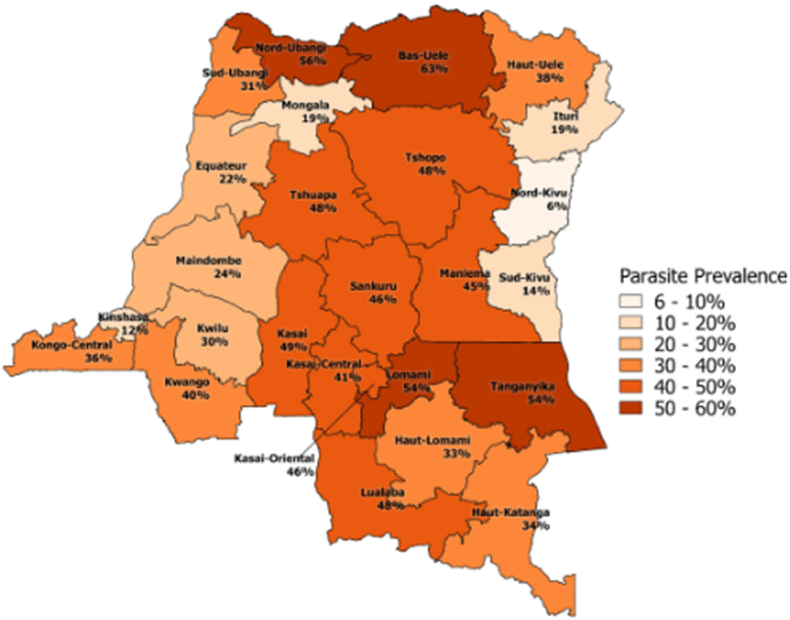
In DR Congo, IPTp3+ implementation is less than the universal coverage and the most of women who attend at least four antenatal care visits receive less than three doses of IPTp [38]. Infants are not covered by preventive chemotherapy strategies (IPTi) as recommended by the WHO. Preventive chemotherapy would help children under five years of age (IPTc) as they are more at risk of malaria morbidity and mortality, and among school-age children (IPTsc) who are a large reservoir of asymptomatic carriers for disease transmission [39]. It has been revealed that IPTc, given every four months combined with timely treatment of febrile malaria illness, has greatly reduced childhood morbidity and mortality related to malaria [40]. In regions with moderate to high transmission, RTS, S/AS01 should be provided to children as part of a comprehensive malaria control strategy in four-dose schedule from 5 months of age [35]. In order to adhere to malaria preventive measures, the promotion of social and behavioural change through education and training to increase the awareness and knowledge of populations such as parents (especially mothers), caregivers, teachers, and healthcare professionals, concerning malaria transmission risks, case management and prevention is essential [41]. The DR Congo's National Strategic Plan 2020–2023 aims to improve the DR Congo population's health status by improving the coverage of epidemiological surveillance and monitoring and evaluation activities. Also, to collaborate with the Direction des Soins de Santé Primaires to make functional and strengthen the capacities of community participation structures in the planning and implementation of malaria control activities, strengthen the behavior change communication for malaria control, and promote the community use of health services [42]. Fig. 1 show the prevalence of Children 6–59 months of age who tested positive for malaria by microscopy (43). Fig. 2 show the Democratic Republic of Congo Malaria Map [44].
Fig. 1.
Prevalence of Children 6 to 59 months of age who tested positive for malaria by microscopy [43].
Fig. 2.
Democratic Republic of Congo Malaria Map [44].
3. Conclusion
The persistent malaria problem in eastern DR Congo's refugee camps necessitates a rapid, broad response. Armed conflict, relocation, and poor living conditions have all contributed to an increased risk of malaria transmission, which disproportionately affects children under the age of five. While organizations such as the World Health Organization and numerous non-governmental groups have attempted to prevent malaria by distributing insecticide-treated nets and drugs, their efforts have fallen short due to the region's instability and a lack of resources. Addressing this crucial issue necessitates a multifaceted approach that includes vector control, improved diagnosis and treatment capabilities, and strengthened local health systems. This technique relies heavily on training healthcare personnel, guaranteeing a regular supply of antimalarial medications, and engaging the local population. Furthermore, effective surveillance and research are required to track disease frequency and resistance tendencies. By implementing these targeted interventions, we can protect the most vulnerable children, reduce malaria-related morbidity and mortality, and go closer to eradicating this regional public health problem.
Ethics approval and consent to participle
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Funding
The authors did not receive any financial support for this work. No funding has been received for the conduct of this study.
Provenance and peer review
Not commissioned, externally peer reviewed.
CRediT authorship contribution statement
Amidu Alhassan: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Lydia Sope Ajala: Formal analysis, Data curation, Conceptualization. Bella Ode: Data curation, Conceptualization. Muhammad Alanjiro: Formal analysis, Data curation, Conceptualization. Sumaira Rehman: Formal analysis, Data curation, Conceptualization. Jones Onesime: Formal analysis, Data curation, Conceptualization. Elie Kihanduka: Conceptualization, Data curation. Christian Tague: Data curation, Formal analysis. Kanza Farhan: Funding acquisition, Formal analysis, Data curation, Conceptualization. Styves Banga: Conceptualization, Data curation, Formal analysis. Excellent Rugendabanga: Data curation, Formal analysis, Funding acquisition. Alvin Manga: Data curation, Formal analysis, Funding acquisition. Gift Joseph Mbwambo: Data curation. Samson Hangi: Formal analysis, Data curation, Conceptualization. Francois Rhugendabanga: Conceptualization, Data curation, Formal analysis. Innocent Mufungizi: Conceptualization, Data curation. Muhammad Furqan: Data curation, Conceptualization. Maher Ali Rusho: Conceptualization, Data curation. Mayar Moustafa Budair: Conceptualization, Data curation. Aymar Akilimali: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that there no conflict of interest.
Acknowledgements
The authors would like to thank the direction of Medical Research Circle (MedReC) of Democratic Republic of the Congo for the realization of this present paper.
Handling Editor: Patricia Schlagenhauf
Contributor Information
Amidu Alhassan, Email: Amidualhassan24@gmail.com.
Lydia Sope Ajala, Email: moshoperede@gmail.com.
Bella Ode, Email: belaode96@gmail.com.
Muhammad Alanjiro, Email: alanjiro2@gmail.com.
Sumaira Rehman, Email: sumairarehman999@gmail.com.
Jones Onesime, Email: jonesonesime@gmail.com.
Elie Kihanduka, Email: kihandukaelie@gmail.com.
Christian Tague, Email: christian.tague@hotmail.com.
Kanza Farhan, Email: kanzafarhan123@gmail.com.
Styves Banga, Email: bangastyves60@gmail.com.
Excellent Rugendabanga, Email: rugex96@gmail.com.
Alvin Manga, Email: alvinmanga3@gmail.com.
Gift Joseph Mbwambo, Email: giftmbwambo7@gmail.com.
Samson Hangi, Email: samson.hangi@hotmail.com.
Francois Rhugendabanga, Email: frncoisrhugenda@gmail.com.
Innocent Mufungizi, Email: mufungiziinm@gmail.com.
Muhammad Furqan, Email: imfuqii@gmail.com.
Maher Ali Rusho, Email: maher.rusho@colorado.edu.
Mayar Moustafa Budair, Email: mayarbudair@gmail.com.
Aymar Akilimali, Email: aymarakilimali@gmail.com.
Medical Research Circle Collaborators:
Malik Olatunde Oduoye, Fabien Balagizi, Chrispin Biamba, Hugues Cakwira, Gentil Rajabu, Hardy Elembwe, Alain Balume, Bonk Muhoza, and Leonard Sironge
References
- 1.World Health Organization . World Health Organisation; 2023. Malaria.https://www.who.int/news-room/fact-sheets/detail/malaria [Google Scholar]
- 2.UN Refugee Agency Democratic republic of the Congo. UN refugee agency. 2022. https://reporting.unhcr.org/operational/operations/democratic-republic-congo
- 3.World Health Organization, W . 2020. Malaria eradication : benefits , future scenarios A report of the Strategic Advisory Group. [Google Scholar]
- 4.UNICEF . 2020. World malaria day 2020; pp. 2018–2020. April. [Google Scholar]
- 5.Global Fund . 2023. Results report 2023. 01; p. 112. [Google Scholar]
- 6.UNICEF . UNICEF; 2019. DRC: 3 million mosquito nets distributed in 20 days.https://www.unicef.org/drcongo/en/stories/drc-3-million-mosquito-nets-distributed-20-days [Google Scholar]
- 7.Debarre A. International Peace Institute; 2022. Hard to reach: providing healthcare in armed conflict. [Google Scholar]
- 8.Azevedo M.J. Historical perspectives on the state of health and health systems in Africa, volume II: the modern era. 2017. The state of health system(s) in Africa: challenges and opportunities; pp. 1–73. [DOI] [Google Scholar]
- 9.Brooks H., Katsuva J., Masumbuko C., Mocanu V., Hawkes M. Use and disuse of malaria bed nets in an internally displaced persons camp in the Democratic Republic of the Congo: a mixed-methods study. PLoS One. 2017;12 doi: 10.1371/journal.pone.0185290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simba D.O., Kakoko D., Nyamhanga T., Mrango Z., Mujinja P. Improving prompt access to malaria diagnostics and treatment in rural remote areas using financial benefit for community health workers in Kilosa district, Tanzania. Res Rep Trop Med. 2018;9:137–146. doi: 10.2147/RRTM.S172944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Worges M., Whitehurst N., Yamo E., Moonga H., Yukich J., Benavente L. Outreach training and supportive supervision for malaria case management in Zambia: the effects of focused capacity building on indicators of diagnostic and clinical performance. Malar J. 2018;17(1):438. doi: 10.1186/s12936-018-2589-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roll Back Malaria RBM partnership to end malaria overview. 2022. https://endmalaria.org/about-us/overview1
- 13.World Bank Congo, dem. Rep. Data. 2023. https://data.worldbank.org/country/CD Retrieved March 25, 2023, from.
- 14.Institute for Health Metrics and Evaluation Democratic republic of the Congo. 2015, September 9. https://www.healthdata.org/democratic-republic-congo Available from. Retrieved Cited June 5, 2024.
- 15.Strategic Advisory Group on Malaria Eradication . A report of the Strategic Advisory Group on Malaria Eradication. Genève : Organisation mondiale de la Santé. Licence: CC BY-NC-SA 3.0 IGO; 2021. Malaria eradication: benefits, future scenarios and feasibility. [Google Scholar]
- 16.Bahizire E., D'Alessandro U., Dramaix M., Dauby N., Bahizire F., Mubagwa K., et al. Malaria and iron load at the first antenatal visit in the rural South Kivu, democratic republic of the Congo: is iron supplementation safe or could it Be harmful? Am J Trop Med Hyg. 2018;98(2):520–523. doi: 10.4269/ajtmh.17-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ngatu N.R., Kanbara S., Renzaho A., Wumba R., Mbelambela E.P., Muchanga S.M., et al. Environmental and sociodemographic factors associated with household malaria burden in the Congo. Malar J. 2019;18(53) doi: 10.1186/s12936-019-2687-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panzi E.K., Okenge L.N., Kabali E.H., Tshimungu F., Dilu A.K., Mulangu F., et al. Geo-climatic factors of malaria morbidity in the democratic republic of Congo from 2001 to 2019. Int J Environ Res Publ Health. 2022;19(3811) doi: 10.3390/ijerph19073811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nundu S.S., Simpson S.V., Arima H., Muyembe J.J., Mita T., Ahuka S., et al. It is time to strengthen the malaria control policy of the democratic republic of Congo and include schools and school-age children in malaria control measures. Pathogens. 2022;11(7):729. doi: 10.3390/pathogens11070729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ntamabyaliro N.Y., Burri C., Lula Y.N., Ishoso D., Engo A.B., Ngale M.A., et al. Knowledge of antimalarials and health seeking behaviour of households in case of suspected malaria in democratic republic of the Congo. Trav Med Infect Dis. 2021;6(3):157. doi: 10.3390/tropicalmed6030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ngimbi N.P., Beckers A., Wery M. Survey of the epidemiological status of malaria in Kinshasa (republic of zaire) in 1980. Ann Soc Belge Med Trop. 1982;62:121–137. [PubMed] [Google Scholar]
- 22.United Nations Department of Economic and Social Affairs, Population Division . 2014. World urbanization prospects: the 2014 revision. [Google Scholar]
- 23.Severe Malaria Observatory Democratic republic of Congo (DRC) https://www.scirp.org/journal/paperinformation?paperid=118733Cited Available from.
- 24.Farhan K., Saeed N., Khan S.R., Tariq B., Ahmed A., Akilimali A. Malaria prevention during pregnancy: implications for maternal and neonatal health in East Africa. New Microbes and New Infections. 2023;55 doi: 10.1016/j.nmni.2023.101186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed A., Mulatu K., Elfu B. Prevalence of malaria and associated factors among under-five children in Sherkole refugee camp, Benishangul-Gumuzregion, Ethiopia. A cross-sectional study. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0246895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wesolowski A., Buckee C., Engø-Monsen K., Metcalf C. Connecting mobility to infectious diseases: the promise and limits of mobile phone data. J Infect Dis. 2016;214:414–420. doi: 10.1093/infdis/jiw273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messenger L., Furnival-Adams J., Pelloquin B., Rowland M. Vector control for malaria prevention during humanitarian emergencies: protocol for a systematic review and meta-analysis. BMJ Open. 2021;11:1–10. doi: 10.1136/bmjopen-2020-046325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eshag H., Elnzer E., Nahied E., Talib M., Mussa A., Muhajir A., et al. Molecular epidemiology of malaria parasite amongst patients in a displaced people's camp in Sudan. Trop Med Health. 2020;48:1–7. doi: 10.1186/s41182-020-0192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aylett J., Gilman R., Hall I., Kennedy D., Evers E., Katta A., et al. Epidemiological modelling in refugee and internally displaced people settlements: challenges and ways forward. BMJ Glob Health. 2022;7:1–10. doi: 10.1136/bmjgh-2021-007822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson J., Doocy S., Haskew C., Spiegel P., Moss W.J. The burden of malaria in post-emergency refugee sites: a retrospective study. Conflict Health. 2011;5:17. doi: 10.1186/1752-1505-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semakula H.M., Liang S., Mukwaya P.I., et al. Determinants of malaria infections among children in refugee settlements in Uganda during 2018–2019. Infect Dis Poverty. 2023;12:31. doi: 10.1186/s40249-023-01090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tatem A., Jia P., Ordanovich D., Falkner M., Huang Z., Howes R., et al. The geography of imported malaria to non-endemic countries: a meta-analysis of nationally reported statistics. Lancet Infect Dis. 2017;17:98–107. doi: 10.1016/S1473-3099(16)30326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization . World Health Organization; Geneva: 2019. Guidelines for malaria vector control; p. 138.https://iris.who.int/handle/10665/310862Cited2024June5 Available from: [PubMed] [Google Scholar]
- 34.President’s Malaria Initiative Democratic republic of the Congo. Malaria operational plan for year 2020. 26 August 2021. https://www.pmi.gov/where-we-work/d-r-congo/Cited
- 35.Bavurhe R.F., Akilimali A., Muhoza B., Biamba C., Oduoye M.O., Masimango G., et al. What are the challenges and the possible solutions to fight Malaria in the Democratic Republic of Congo? New Microbes New Infect. 2023 Jun 14;54 doi: 10.1016/j.nmni.2023.101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swana E.K., Makan G.Y., Mukeng C.K., Mupumba H.I., Kalaba G.M., Luboya O.N., et al. Feasibility and implementation of community-based malaria case management with integrated vector control in the Democratic Republic of Congo. Malar J. 2016;15(1):413. doi: 10.1186/s12936-016-1475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health, Organization World malaria report 2021. 2021. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021Cited2024Jun4 Available from:
- 38.Pons-Duran C., Llach M., Sacoor C., Sanz S., Macete E., Arikpo I., et al. Coverage of intermittent preventive treatment of malaria in pregnancy in four sub-Saharan countries: findings from household surveys. Int J Epidemiol. 2021;50(2):550–559. doi: 10.1093/ije/dyaa233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Intermittent preventive treatment of malaria delivered to primary schoolchildren provided effective individual protection in Jinja, Uganda: secondary outcomes of a cluster-randomized trial (START-IPT) - PubMed. Available from: https://pubmed.ncbi.nlm.nih.gov/31533845/Cited2024June5. [DOI] [PMC free article] [PubMed]
- 40.Mukherjee D., Dhar R., Devi A., Gorai S., Muthusamy R., Ganesh P.S., et al. Exosome-based malaria vaccine, a miracle of nanomedicine. Int J Surg: Glob Health. 2023;6(3) doi: 10.1097/GH9.0000000000000141. [DOI] [Google Scholar]
- 41.Ahorlu C.K., Koram K.A., Seake-Kwawu A., Weiss M.G. Two-year evaluation of Intermittent Preventive Treatment for Children (IPTc) combined with timely home treatment for malaria control in Ghana. Malar J. 2011;10:127. doi: 10.1186/1475-2875-10-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akilimali A., Bisimwa C., Aborode A.T., Biamba C., Sironge L., Balume A., et al. Self-medication and anti-malarial drug resistance in the democratic republic of the Congo (DRC): a silent threat. Trop Med Health. 2022;50(1):73. doi: 10.1186/s41182-022-00466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.UNICEF Democratic republic of Congo multiple indicator cluster survey. Sustainability. 2019;11(1):1–14. doi: 10.1016/j.regsciurbeco.2008.06.005%0A. http://scioteca.caf.com/bitstream/handle/123456789/1091/RED2017-Eng-8ene.pdf?sequence=12&isAllowed=y%0A https://www.researchgate.net/publication/305320484_SISTEM_PEMBETUNGAN_TERPUSAT_STRATEGI_MELESTARI [DOI] [Google Scholar]
- 44.Public Health Scotland . Fitfortravel.nhs.UK. 2024. Democratic republic of the Congo malaria Map - fit for travel.https://www.fitfortravel.nhs.uk/destinations/africa/democratic-republic-of-congo/democratic-republic-of-the-congo-malaria-map [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.