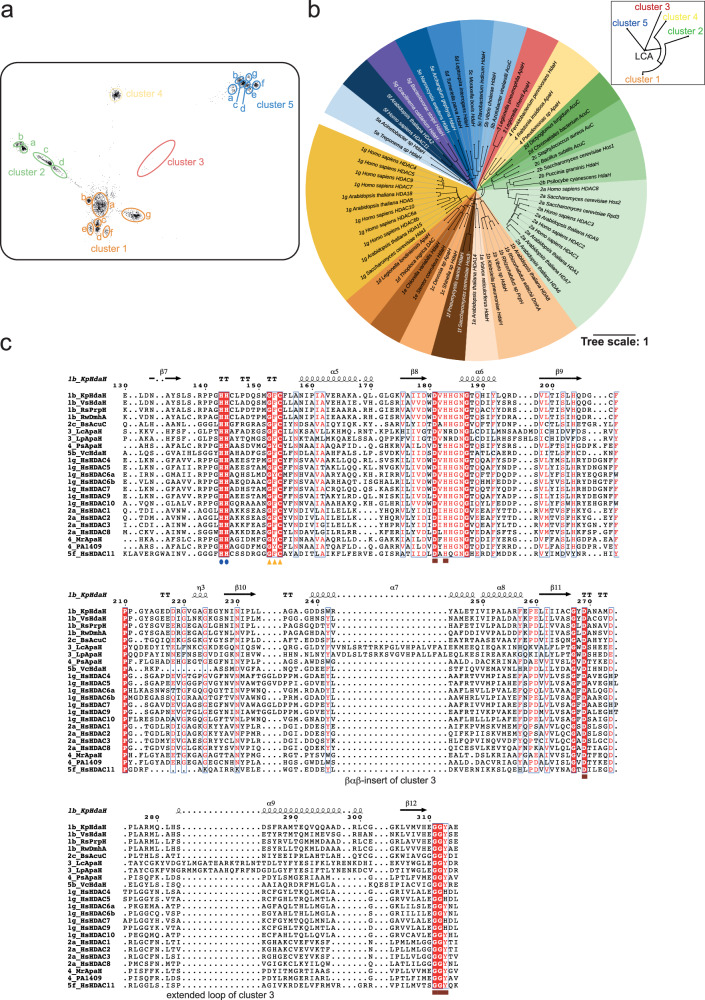
Fig. 1. Bacteria encode a plethora of Zn2+-dependent deacylases.
a Bacterial deacylases can be classified into five clusters, some with several sub-clusters. A Generalized Profile (GP) was constructed from a multiple sequence alignment (MSA) of classical deacetylases, which resulted in thousands of sequences upon screening the UniProt database. Clustering was done using the program clans (cluster analysis of sequences). Each sequence is represented as a dot on a two-dimensional plane, i.e. their 2D-distances correspond to sequence similarities. This resulted in a total of five major clusters (clusters 1–5). The clusters 1, 2, and 5 are subdivided into several sub-clusters, i.e., 1a–1g, 2a–2d, and 5a–5g. b Phylogenetic tree of classical Zn2+-dependent deacylases of selected bacterial deacylases representing all clusters. The human enzymes, the deacylases of S. cerevisiae and the classical deacylases from A. thaliana are highlighted. All human enzymes are categorized in cluster 1 (HDAC class IIa and IIb), cluster 2 (HDACs class I), and cluster 5 (HDAC11). The closeup shows the development of the clusters from the LCA (last common ancestor). The unrooted phylogenetic tree was created with iTOL using a multiple sequence alignment of the catalytic domains (deleted >90% of gaps) created by MAFFT. c Amino acid sequence alignment of selected classical Zn2+-dependent deacylases. The catalytic residues are totally conserved in enzymes from Homo sapiens and from bacteria. Shown are representative human enzymes of each class and bacterial enzymes representing each cluster (1–5) and the enzymes PA1409 and MrApaH characterized earlier117,124. The numbering and the secondary structure elements were shown for KpHdaH (1b) above the alignment. Blue circles: double-His motif, with the second His acting as catalytic base/acid (KpHdaH: His143-His144); yellow triangles: conserved GFC-motif lining the substrate binding channel; brown squares: Asp-His-Asp for coordination of the catalytic Zn2+-ion; brown rectangle: (E/S/G)GGY-motif lining the foot pocket for substrate release and the catalytic Tyr (KpHdaH: Tyr313) important for orientation/polarization of the acetyl-group and for stabilization of the negative charged oxygen arising in the tetrahedral intermediate. The MSA was conducted with the T-Coffee algorithm and ESPript version 3.0 was used to create the figure212,213,218,219.