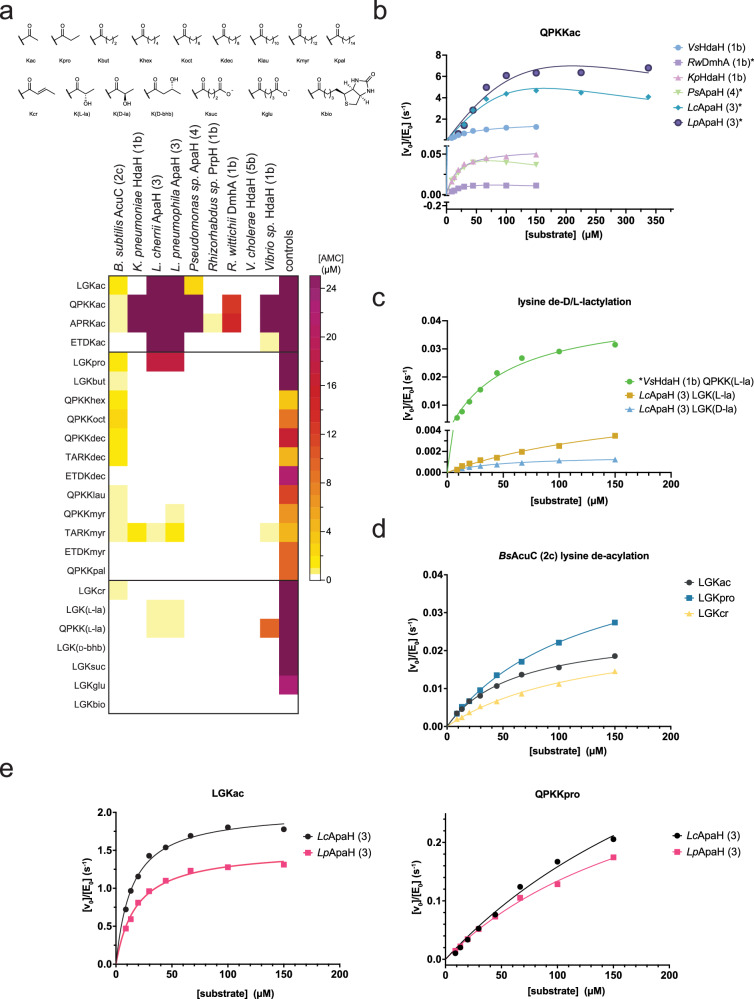
Fig. 3. Pre-screening to uncover acyl-chain preferences of bacterial deacylases.
a Pre-screening of selected bacterial deacylases to assess their preferences for different acyl-chain types. A Fluor-de-Lys assay-based screening was performed using histone H3 (APRKacyl, H315-18 or TARKacyl, H36-9), histone H4 (LGKacyl, H410-12), p53 (QPKKacyl, p53317-320) and DLAT derived peptide sequences. Depicted is the conversion of peptide in released [AMC] in µM. Positive controls as described in the “Methods” section. The graph depicts the means of both recorded independent replicates (n = 2). Source data are provided as Source Data file. b Michaelis–Menten kinetics for bacterial deacetylases (discontinuous assay). All selected bacterial deacylases showing robust deacetylase activity were summarized (QPKKac, p53317-320). Notably, for RwDmhA (1b), PsApaH (4), and LcApaH (3)/LpApaH (3) we observed substrate inhibition at higher substrate concentration. *: Data adjusted to kinetics with substrate inhibition at high concentration. The experiments were performed in two independent replicates (n = 2). Data are presented as means. Source data are provided as Source Data file. c Michaelis–Menten kinetics for the delactylases VsHdaH (1b) and LcApaH (3). Notably, for VsHdaH (1b) we observed a stereoselectivity toward de-L-lactylation (QPKKl-la, p53317-320), while the LcApaH (3) converts both stereoisomers acting as de-d/l-lactylase (LGKd-la/l-la, H410-12). The experiment was done in continuous assay format for VsHdaH (1b) and LcApaH (3). The * indicates the discontinuous assay format used for VsHdaH (1b). The experiments were performed in two independent replicates (n = 2). Data are presented as means. Source data are provided as Source Data file. d Michaelis–Menten kinetics for the deacylase activity for BsAcuC (2c) (continuous assay). BsAcuC (2c) is active as deacetylase, depropionylase, and decrotonylase (LGKacyl, H410-12). The experiments were performed in two independent replicates (n = 2) in a continuous assay format. Data are presented as means. Source data are provided as Source Data file. e Michaelis–Menten kinetics for the deacetylase and depropionylase activity of LpApaH (3) and LcApaH (3) (deactylase: continuous assay format; depropionylase: discontinuous assay format). LpApaH (3) and LcApaH (3) are active deacetylases (LGKac, H410-12) and depropionylases (QPKKacyl, p53317-320). The experiments were performed in two independent replicates (n = 2). Data are presented as means. Source data are provided as Source Data file.