Abstract
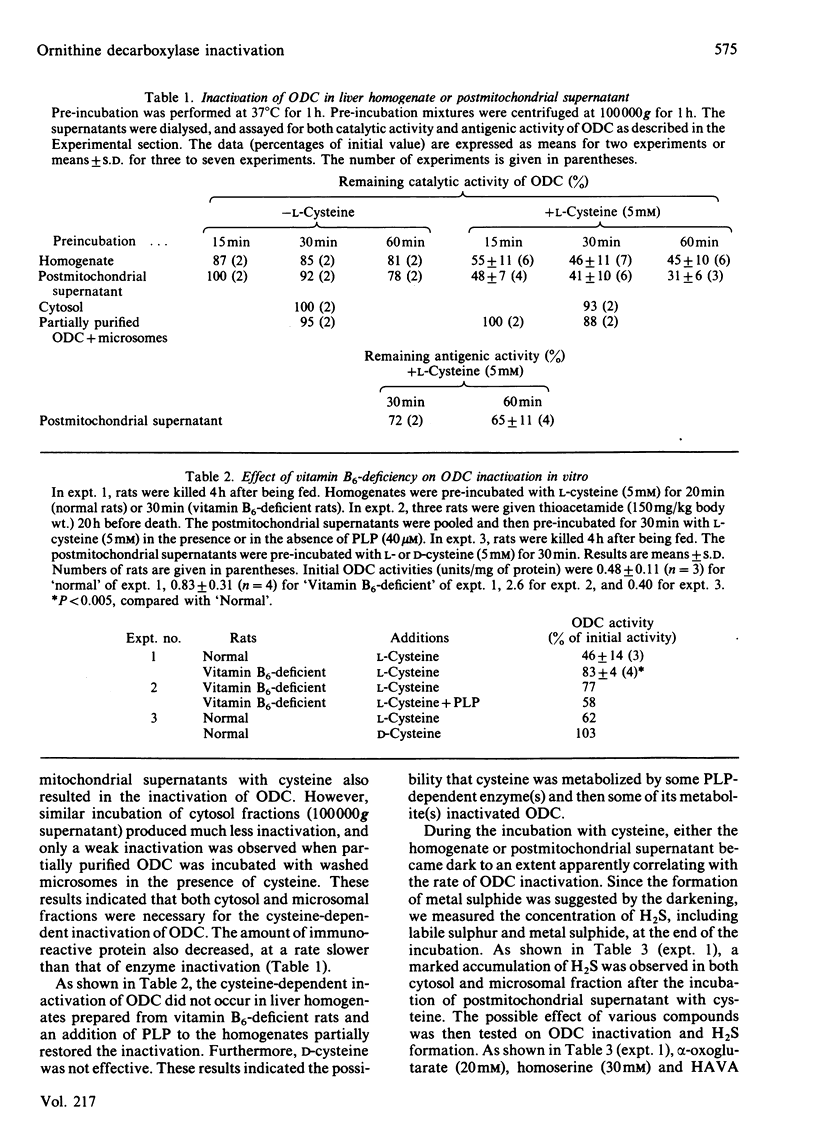
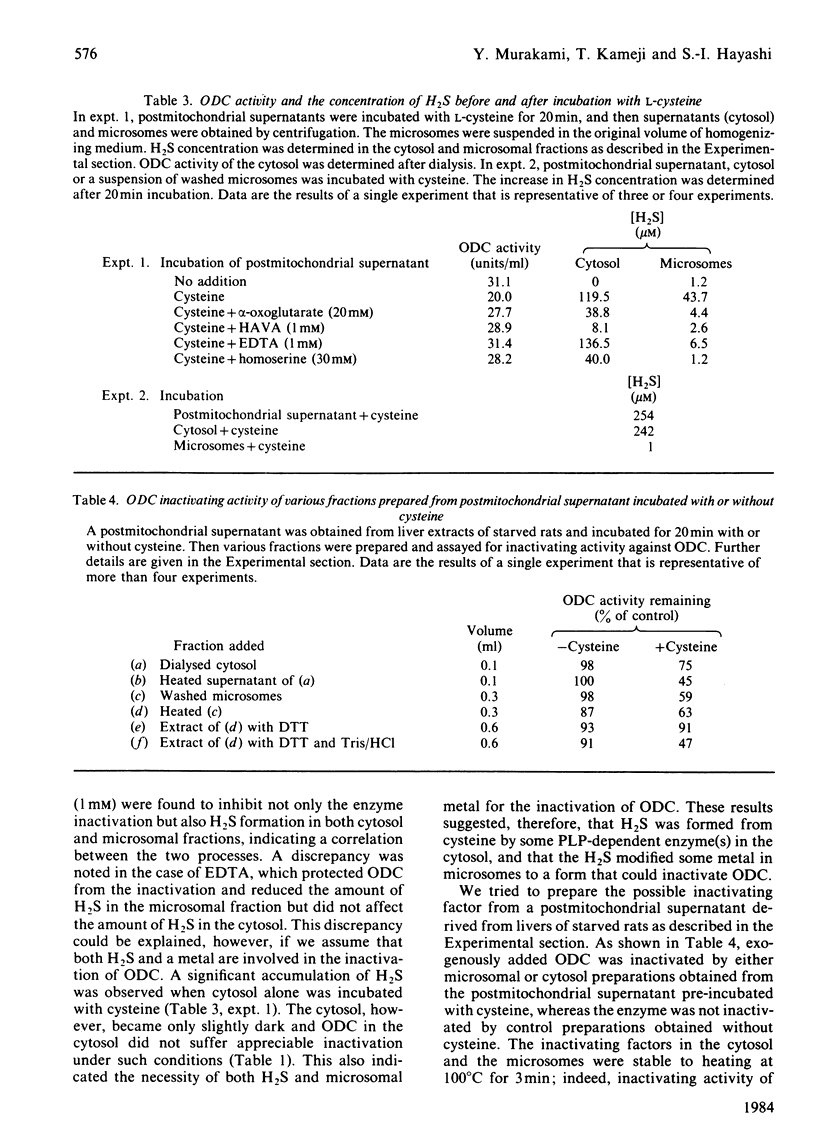
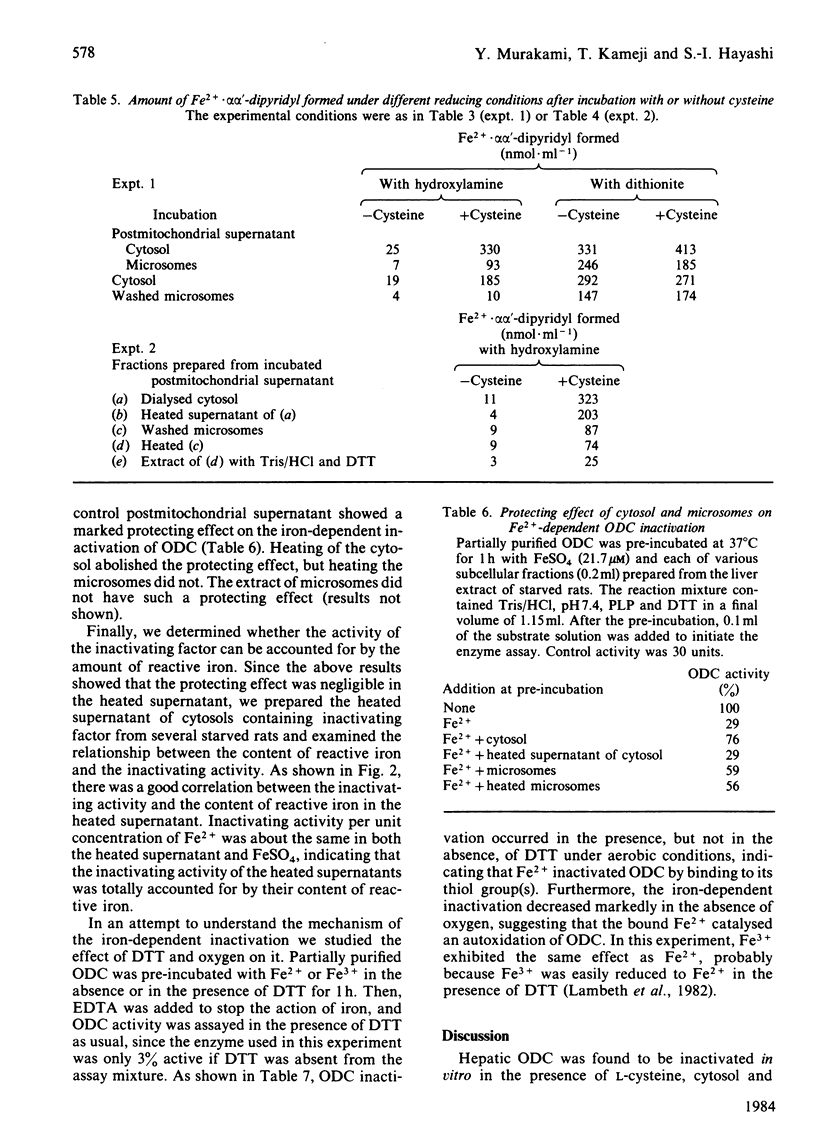
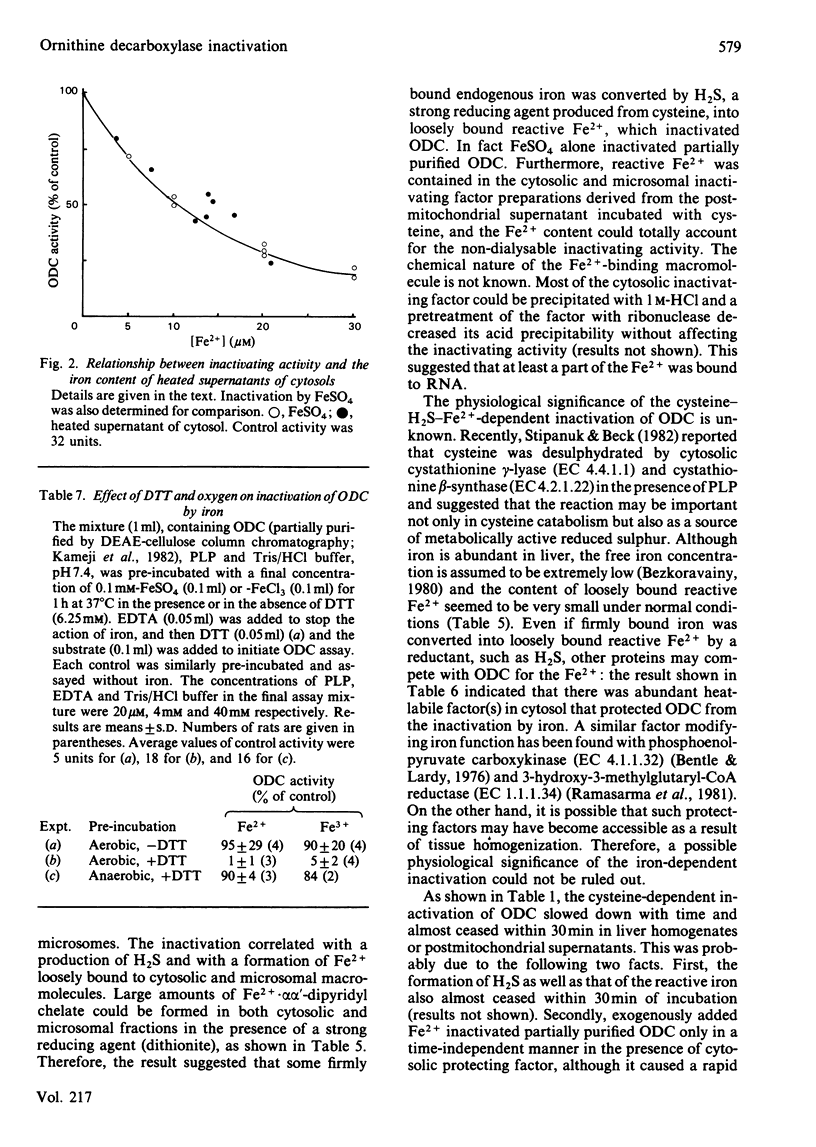
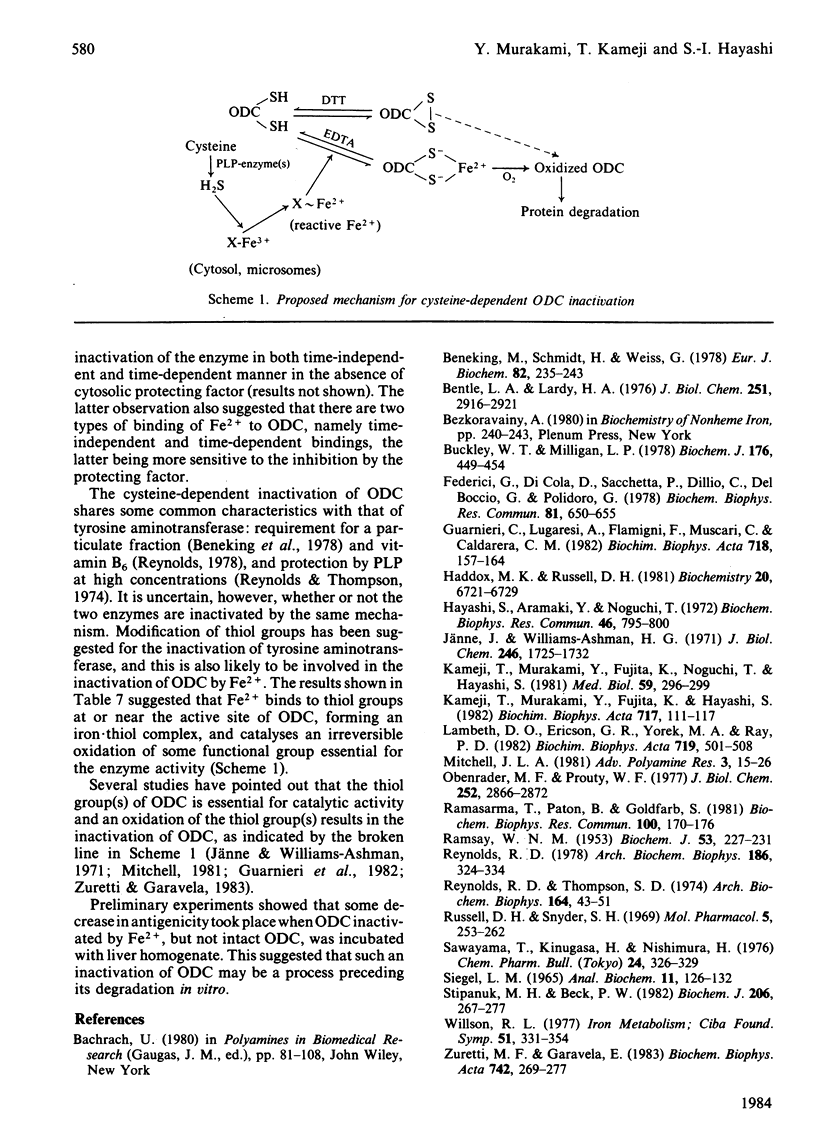
When rat liver homogenate or its postmitochondrial supernatant was incubated with L-cysteine, but not D-cysteine, ornithine decarboxylase (ODC) lost more than half of its catalytic activity within 30 min and, at a slower rate, its immunoreactivity. The inactivation correlated with production of H2S during the incubation. These changes did not occur in liver homogenates from vitamin B6-deficient rats. A heat-stable inactivating factor was found in both dialysed cytosol and washed microsomes obtained from the postmitochondrial supernatant incubated with cysteine. The microsomal inactivating factor was solubilized into Tris/HCl buffer, pH 7.4, containing dithiothreitol. Its absorption spectrum in the visible region resembled that of Fe2+ X dithiothreitol in Tris/HCl buffer. On the other hand FeSO4 inactivated partially purified ODC in a similar manner to the present inactivating factor. During the incubation of postmitochondrial supernatant with cysteine, there was a marked increase in the contents of Fe2+ loosely bound to cytosolic and microsomal macromolecules. Furthermore, the content of such reactive iron in the inactivating factor preparations was enough to account for their inactivating activity. These data suggested that H2S produced from cysteine by some vitamin B6-dependent enzyme(s) converted cytosolic and microsomal iron into a reactive loosely bound form that inactivated ODC.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beneking M., Schmidt H., Weiss G. Subcellular distribution of a factor inactivating tyrosine aminotransferase. Study of its mechanism and relationship to different forms of the enzyme. Eur J Biochem. 1978 Jan 2;82(1):235–243. doi: 10.1111/j.1432-1033.1978.tb12016.x. [DOI] [PubMed] [Google Scholar]
- Bentle L. A., Lardy H. A. Interaction of anions and divalent metal ions with phosphoenolpyruvate carboxykinase. J Biol Chem. 1976 May 25;251(10):2916–2921. [PubMed] [Google Scholar]
- Buckley W. T., Milligan L. P. Participation of cysteine and cystine in inactivation of tyrosine aminotransferase in rat liver homogenates. Biochem J. 1978 Nov 15;176(2):449–454. doi: 10.1042/bj1760449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici G., Di Cola D., Sacchetta P., Di Ilio C., Del Boccio G., Polidoro G. Reversible inactivation of tyrosine aminotransferase from guinea pig liver by thiol and disulfide compounds. Biochem Biophys Res Commun. 1978 Mar 30;81(2):650–655. doi: 10.1016/0006-291x(78)91585-1. [DOI] [PubMed] [Google Scholar]
- Guarnieri C., Lugaresi A., Flamigni F., Muscari C., Caldarera C. M. Effect of oxygen radicals and hyperoxia on rat heart ornithine decarboxylase activity. Biochim Biophys Acta. 1982 Oct 8;718(2):157–164. doi: 10.1016/0304-4165(82)90214-8. [DOI] [PubMed] [Google Scholar]
- Haddox M. K., Russell D. H. Ornithine decarboxylase from calf liver. Purification and properties. Biochemistry. 1981 Nov 10;20(23):6721–6729. doi: 10.1021/bi00526a030. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Aramaki Y., Noguchi T. Diurnal change in ornithine decarboxylase activity of rat liver. Biochem Biophys Res Commun. 1972 Jan 31;46(2):795–800. doi: 10.1016/s0006-291x(72)80211-0. [DOI] [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. On the purification of L-ornithine decarboxylase from rat prostate and effects of thiol compounds on the enzyme. J Biol Chem. 1971 Mar 25;246(6):1725–1732. [PubMed] [Google Scholar]
- Kameji T., Murakami Y., Fujita K., Hayashi S. Purification and some properties of ornithine decarboxylase from rat liver. Biochim Biophys Acta. 1982 Jul 16;717(1):111–117. doi: 10.1016/0304-4165(82)90387-7. [DOI] [PubMed] [Google Scholar]
- Kameji T., Murakami Y., Fujita K., Noguchi T., Hayashi S. Rat liver ornithine decarboxylase purification and some immunochemical studies. Med Biol. 1981 Dec;59(5-6):296–299. [PubMed] [Google Scholar]
- Lambeth D. O., Ericson G. R., Yorek M. A., Ray P. D. Implications for in vitro studies of the autoxidation of ferrous ion and the iron-catalyzed autoxidation of dithiothreitol. Biochim Biophys Acta. 1982 Dec 17;719(3):501–508. doi: 10.1016/0304-4165(82)90239-2. [DOI] [PubMed] [Google Scholar]
- Obenrader M. F., Prouty W. F. Production of monospecific antibodies to rat liver ornithine decarboxylase and their use in turnover studies. J Biol Chem. 1977 May 10;252(9):2866–2872. [PubMed] [Google Scholar]
- RAMSAY W. N. M. The determination of iron in blood plasma or serum. Biochem J. 1953 Jan;53(2):227–231. doi: 10.1042/bj0530227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasarma T., Paton B., Goldfarb S. Inactivation of 3-hydroxy-3-methylglutaryl CoA reductase by Fs++ and a cytosolic protein. Biochem Biophys Res Commun. 1981 May 15;100(1):170–176. doi: 10.1016/s0006-291x(81)80078-2. [DOI] [PubMed] [Google Scholar]
- Reynolds R. D., Thompson S. D. Irreversible inactivation of rat liver tyrosine aminotransferase. Arch Biochem Biophys. 1974 Sep;164(1):43–51. doi: 10.1016/0003-9861(74)90006-x. [DOI] [PubMed] [Google Scholar]
- Reynolds R. D. Vitamin B-6 requirement for irreversible inactivation of rat liver tyrosine aminotransferase. Arch Biochem Biophys. 1978 Mar;186(2):324–334. doi: 10.1016/0003-9861(78)90442-3. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H. Amine synthesis in regenerating rat liver: extremely rapid turnover of ornithine decarboxylase. Mol Pharmacol. 1969 May;5(3):253–262. [PubMed] [Google Scholar]
- SIEGEL L. M. A DIRECT MICRODETERMINATION FOR SULFIDE. Anal Biochem. 1965 Apr;11:126–132. doi: 10.1016/0003-2697(65)90051-5. [DOI] [PubMed] [Google Scholar]
- Sawayama T., Kinugasa H., Nishimura H. Syntheses of ornithine decarboxylase inhibitors: D- and DL-alpha-hydrazinoornithine. Chem Pharm Bull (Tokyo) 1976 Feb;24(2):326–329. doi: 10.1248/cpb.24.326. [DOI] [PubMed] [Google Scholar]
- Stipanuk M. H., Beck P. W. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982 Aug 15;206(2):267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuretti M. F., Gravela E. Studies on the mechanisms of ornithine decarboxylase in vitro inactivation. Biochim Biophys Acta. 1983 Jan 26;742(2):269–277. doi: 10.1016/0167-4838(83)90311-4. [DOI] [PubMed] [Google Scholar]


