Abstract
BACKGROUND
Monozygotic (MZ) twins are believed to arise from the fission of a single fertilized embryo at different stages. Monochorionic MZ twins, who share one chorion, originate from the splitting of the inner cell mass (ICM) within a single blastocyst. In the classic model for dichorionic MZ twins, the embryo splits before compaction, developing into two blastocysts. However, there are a growing number of ART cases where a single blastocyst transfer results in dichorionic MZ twins, indicating that embryo splitting may occur even after blastocyst formation.
OBJECTIVE AND RATIONALE
For monochorionic MZ twins, we conducted a comprehensive analysis of the cellular mechanisms involved in ICM splitting, drawing from both ART cases and animal experiments. In addition, we critically re-examine the classic early splitting model for dichorionic MZ twins. We explore cellular mechanisms leading to two separated blastocysts in ART, potentially causing dichorionic MZ twins.
SEARCH METHODS
Relevant studies including research articles, reviews, and conference papers were searched in the PubMed database. Cases of MZ twins from IVF clinics were found by using combinations of terms including ‘monozygotic twins’ with ‘IVF case report’, ‘ART’, ‘single embryo transfer’, or ‘dichorionic’. The papers retrieved were categorized based on the implicated mechanisms or as those with unexplained mechanisms. Animal experiments relating to MZ twins were found using ‘mouse embryo monozygotic twins’, ‘mouse 8-shaped hatching’, ‘zebrafish janus mutant’, and ‘nine-banded armadillo embryo’, along with literature collected through day-to-day reading. The search was limited to articles in English, with no restrictions on publication date or species.
OUTCOMES
For monochorionic MZ twins, ART cases and mouse experiments demonstrate evidence that a looser ICM in blastocysts has an increased chance of ICM separation. Physical forces facilitated by blastocoel formation or 8-shaped hatching are exerted on the ICM, resulting in monochorionic MZ twins. For dichorionic MZ twins, the classic model resembles artificial cloning of mouse embryos in vitro, requiring strictly controlled splitting forces, re-joining prevention, and proper aggregation, which allows the formation of two separate human blastocysts under physiological circumstances. In contrast, ART procedures involving the transfer of a single blastocysts after atypical hatching or vitrified-warmed cycles might lead to blastocyst separation. Differences in morphology, molecular mechanisms, and timing across various animal model systems for MZ twinning can impede this research field. As discussed in future directions, recent developments of innovative in vitro models of human embryos may offer promising avenues for providing fundamental novel insights into the cellular mechanisms of MZ twinning during human embryogenesis.
WIDER IMPLICATIONS
Twin pregnancies pose high risks to both the fetuses and the mother. While single embryo transfer is commonly employed to prevent dizygotic twin pregnancies in ART, it cannot prevent the occurrence of MZ twins. Drawing from our understanding of the cellular mechanisms underlying monochorionic and dichorionic MZ twinning, along with insights into the genetic mechanisms, could enable improved prediction, prevention, and even intervention strategies during ART procedures.
REGISTRAITON NUMBER
N/A.
Keywords: monozygotic twins, assisted reproduction, embryo development, chorion, inner cell mass, blastocyst cavitation, zona pellucida, assisted hatching
Graphical abstract
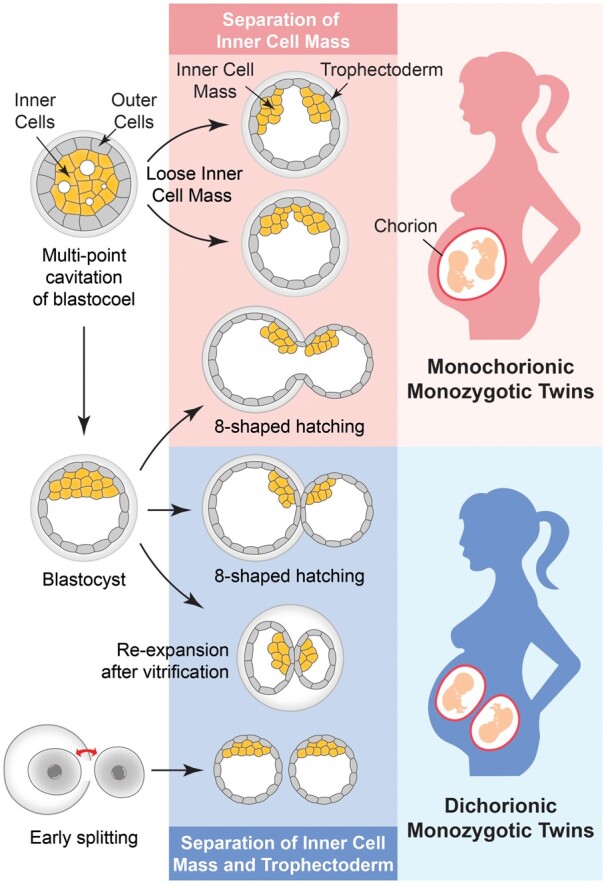
Splitting of the inner cell mass within a blastocyst leads to monochorionic monozygotic twins; if one embryo splits into two blastocysts, dichorionic monozygotic twins develop.
Introduction
In the last four decades, the incidence of twin pregnancies has seen a notable rise worldwide (Imaizumi 2003; Tandberg et al. 2007; Martin et al. 2012), largely attributed to advancements in ART (Aston et al. 2008). Twins can be of two types: dizygotic twins, resulting from the fertilization of two oocytes developing into two distinct embryos (Hoekstra et al. 2008), and monozygotic (MZ) twins, which are a natural occurrence of identical individuals originating from a single fertilized embryo (Corner 1955; Hall 2003; McNamara et al. 2016). Twin pregnancies pose high risks to both the fetuses and the mother, including twin-to-twin transfusion syndrome, twin reversed arterial perfusion sequence, preterm birth, vanishing twins, and various gestational complications (Pinborg 2005; Corsello and Piro 2010; Waszak et al. 2017; Vitucci et al. 2020). In some cases, they can even result in the birth of conjoined twins (Johnston 2001; Kaufman 2004). Whereas the incidence of dizygotic twins can be reduced through single embryo transfer (De Sutter 2006), preventing MZ twins remains challenging because the exact cause of MZ twinning is not fully understood.
For over 100 years, there has been a longstanding effort to unravel the mysteries surrounding the formation of MZ twins, dating back to the early last century (Corner 1922). This extensive history has been comprehensively reviewed recently (Herranz 2015). However, the main limitation of MZ twin studies has been the lack of a suitable mammalian model that consistently exhibits a high rate of MZ twin pregnancies, except for the nine-banded armadillo, which remains the only known animal capable of naturally producing identical quadruplets (Enders 2002; Blickstein and Keith 2007). Despite mouse embryos showing some resemblance to human twin development after manual intervention in vitro (Landeira et al. 2015; Yan et al. 2015; Onodera et al. 2017), naturally occurring MZ twins in mice are rare and most likely cannot survive to birth probably due to lower cell number compared to normal embryos (McLaren et al. 1995). Ethical regulations surrounding human embryo studies further restrict the exploration of MZ twins. Only recently, with the rapid advancements and the widespread use of ART, the real-time observation of human embryos in vitro before implantation has become more feasible (Sutherland et al. 2019; Sciorio and Meseguer 2021; Matorras et al. 2023). The natural rate of MZ twins is about 0.4% (Murphy and Hey 1997), but higher rates of MZ twins have been reported amongst ART cases compared with natural pregnancies, ranging from 0.72% to 5% (Behr et al. 2000; Toledo 2005; Vitthala et al. 2009; Knopman et al. 2010; Papanikolaou et al. 2010; Sharara and Abdo 2010; Kawachiya et al. 2011; Vela et al. 2011; Luke et al. 2014; Mateizel et al. 2016; Parazzini et al. 2016). Thus, studies on MZ twinning during ART provide valuable clues to explore the unknown mechanisms involved (Aston et al. 2008).
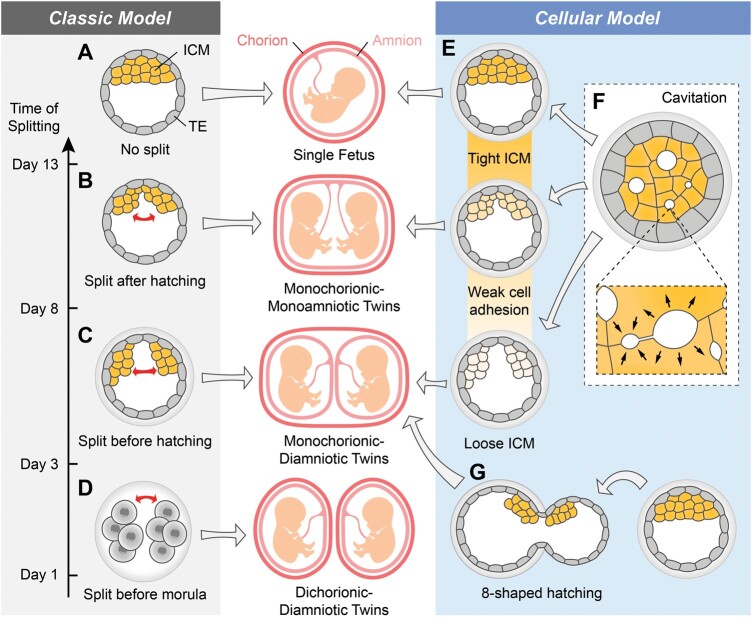
MZ twins are classified based on the number of amnion and chorion, which are membranes that surround and protect the fetus during the pregnancy. In humans, the chorion is primarily developed from the trophectoderm (TE), which constitutes the outer cells of a blastocyst, while the pluripotent inner cell mass (ICM) of a blastocyst mainly contributes to all tissues of the fetus (Fig. 1A) (Rossant and Tam 2022). It is widely believed that if the ICM splits into two groups within one blastocoel surrounded by TE at the blastocyst stage or later, it results in monochorionic MZ twins who share one chorion (Fig. 1B and C) (Hall 2003; McNamara et al. 2016). On the other hand, dichorionic MZ twins result from the separation of both the ICM and TE. This separation is classically thought to arise from the splitting of blastomeres before the morula stage, during the initial 3 days of human embryo development (Fig. 1D) (Hall 2003; McNamara et al. 2016). This leads to the development of two separate blastocysts, giving rise to MZ twins, each with its individual chorion. Recent ART cases, however, diverge from this long-held belief, reporting that a single blastocyst transfer can lead to dichorionic MZ twin pregnancy, which suggests late splitting after the blastocyst stage (Klein et al. 2005; Sundaram et al. 2018; Dirican and Olgan 2021; Semrl et al. 2023).
Figure 1.
Classic model for different types of monozygotic (MZ) twinning (left) and cellular model for monochorionic MZ twin formation (right). (A) During human embryo development, the two cell lineages at the blastocyst stage, the inner cell mass (ICM, yellow) and the trophectoderm (TE, gray), primarily develop into the fetus and the chorion, respectively. (B and C) Monochorionic MZ twins share one chorion, and in most cases, they have their own amnion (third row) but in rare case, they can also share one amnion (second row). Classically, monochorionic MZ twins are formed when the ICM undergoes splitting before or after the blastocyst hatching. (D) Dichorionic MZ twins result from early embryo splitting before the morula stage. (E) Looseness of the ICM can occur during (F) the multi-point initiation of cavitation and there is subsequent accumulation into a single-dominant blastocoel (inset). (G) The 8-shaped hatching blastocysts are likely to undergo ICM separation when the ICM is positioned near the hatching point.
In this review, we will present evidenced cellular mechanisms of monochorionic and dichorionic MZ twin formation, based on recently reported ART studies as well as mouse embryo research, to propose a new model for the generation of MZ twins, which could potentially contribute to a reduction in the occurrence of MZ twin pregnancies during ART, thus lowering the health risks for both mother and fetus.
Methods
Cases of MZ twins from IVF clinics were searched in the PubMed database using various combinations of terms, including ‘monozygotic twins’ along with ‘IVF case report’, ‘ART’, ‘single embryo transfer’, ‘assisted hatching’, or ‘dichorionic’. These papers were subsequently categorized through careful reading based on the potential mechanisms implicated in the cases or those with unexplained mechanisms. Additionally, animal experiments related to MZ twins were searched using terms such as ‘mouse embryo monozygotic twins’, ‘mouse 8-shaped hatching’, ‘zebrafish janus mutant’, ‘nine-banded armadillo embryo’, and ‘blastocyst bisection’. Other papers suggesting mechanisms of MZ twins were collected through regular reading of literature.
Cellular mechanism of monochorionic monozygotic twinning
It is widely accepted that the separation of the ICM at blastocyst stage or after implantation leads to the development of monochorionic MZ twins (Fig. 1B and C). This is supported by documented cases of human blastocysts containing two separate groups of ICMs in a single blastocoel (Meintjes et al. 2001; Mio and Maeda 2008; Noli et al. 2015), and even triple ICMs (Lee et al. 2008), leading to monochorionic twin or triplet pregnancies respectively. Similarly, in mouse studies, blastocysts with double ICMs (Chida 1990; Waheed 2009) or even implanted embryos with two egg cylinder cups (Hsu and Gonda 1980; Waheed 2009) have been observed in vitro. To understand the cellular mechanism underlying these processes, the key questions to address are: (i) the character of ICM splitting and (ii) the nature of the physical force responsible for the splitting.
Looseness of the inner cell mass: reason for splitting
The classic fission model led to speculations that deficient cell adhesion might be the cause, and a potential role of the cell adhesion molecule E-cadherin in the formation of MZ twins was proposed (Bamforth et al. 2003). In assisted reproduction, birth rates of MZ twins increased from 0.38% to 1.38% when blastocysts with a poorer grade of ICM quality were transferred, which include those with a looser ICM (Otsuki et al. 2016; Eliasen et al. 2021).
This theory was supported by a mouse experiment which was not originally designed to study MZ twins but has profound implications (Landeira et al. 2015). Mouse embryonic stem cells (mESCs) that lacked Jarid2 (Jumonji, AT-rich interactive domain 2), a component of the polycomb repressor complex 2, showed a significant reduction in the level of E-cadherin and other genes controlling cell adhesion. Interestingly, when Jarid2-null mESCs were injected into the blastocoel of mouse embryos, multiple ICMs were observed in a single blastocyst. Furthermore, the absence of Jarid2 upregulated ICM-lineage marker Nanog and downregulated planar cell polarity signaling genes Wnt9a, Prickle1, and Fzd2. Notably, injection of mESCs overexpressing Nanog or mESCs depleted of Wnt9a, Prickle1, and Fzd2 also resulted in two or more ICMs in ∼35–48% of blastocysts. Thus, reduced cell adhesion and a looser ICM are most likely significant factors contributing to the splitting of the ICM and consequently might lead to monochorionic MZ twinning (Fig. 1E).
The physical force that splits the inner cell mass
Cavitation of the blastocyst
Extended embryo culture and blastocyst transfer, as compared to the transfer of earlier cleavage-stage embryos, are significant factors contributing to a higher rate of MZ twins during ART procedures (Jain et al. 2004; Skiadas et al. 2008; Kawachiya et al. 2011; Ding et al. 2018; Hviid et al. 2018; Liu et al. 2018a; Busnelli et al. 2019). During the formation of the blastocoel before blastocyst transfer in ART, the intermittent collapse and re-expansion process can potentially lead to the separation of the ICM in human embryos (Payne et al. 2007; Mio and Maeda 2008). It was further verified in a human in vitro model for monochorionic twins that the separation of the ICM can happen during cavitation (Luijkx et al. 2024). Thus, the nature of the blastocyst cavitation process may offer valuable insights into the mechanisms underlying the splitting of the ICM, contributing to the occurrence of MZ twins.
While there are no more in-depth studies available on human embryos, research in mice has shown that the initiation of blastocoel formation in mouse preimplantation embryos does not start at a single point but rather begins from hundreds of micrometer-sized lumens formed between cell–cell junctions through hydraulic fracturing (Fig. 1F) (Dumortier et al. 2019). These small water-filled pockets gradually release their content, eventually leading to the formation of a single-dominant lumen, the blastocoel. When maternal mutant embryos lacking the cell adhesion molecule Cadherin 1 (Cdh1) were combined with wildtype embryos to form chimera embryos, the final blastocoel lumen was collected alongside the Cdh1 knockout cells. This suggests that the direction of lumen accumulation tends to separate regions with lower cell–cell contacts.
Altogether, if the inner cells of the preimplantation embryo, undergoing blastocoel formation, are loosely connected, they are more likely to be separated through multipoint cavitation and the accumulation of fluids. This acts as a physical force that splits the ICM into two or three distinct groups in one blastocyst, ultimately resulting in the formation of MZ twins who share a chorion (Fig. 1E and F).
8-Shaped hatching
In ART, assisted hatching is widely used to help an embryo escape from the zona pellucida (ZP), thereby promoting its progression toward implantation. Various techniques of ZP manipulation can assist hatching, including mechanical dissection, drilling, and thinning (Cohen 1991; Hammadeh et al. 2011; Schimmel et al. 2014). While assisted hatching is not always found to be significantly associated with MZ twinning (Sills et al. 2000; Elizur et al. 2004; Vitthala et al. 2009; Wu et al. 2014; Gu et al. 2018; Busnelli et al. 2019), its potential contribution to MZ twinning during ART procedures is controversially discussed (Saito et al. 2000; Alikani et al. 2003).
When an artificial small hatching slit is created on the ZP by mechanical assisted hatching or ZP drilling, the blastocyst will hatch through this opening, taking on a shape resembling the number ‘8’ (Fig. 1G). In an IVF case, time-lapse live imaging of an 8-shaped hatching human blastocyst revealed that the ICM situated near the hatching point passed through this hatching hole in the blastocyst and divided into multiple parts (Fig. 1G) (Sutherland et al. 2019). One part of the ICM remained inside the ZP, while the other two parts were observed outside, and would thus result in a monochorionic triplet pregnancy. During such 8-shaped hatching events, some of the ICM cells near the hatching point may undergo apoptosis, and the pressure from the narrow gap on the ZP separates the ICM into distinct groups (Ménézo and Sakkas 2002).
Mouse experiments have also provided support for this phenomenon, showing that 8-shaped hatching increases the separation of the ICM at blastocyst stage (Yan et al. 2015; Onodera et al. 2017). This 8-shaped hatching occurs in over 20% of mouse embryos in vitro (Yan et al. 2015). The relative position between the hatching point and ICM has been demonstrated to be vital for ICM separation during 8-shaped hatching, similar to human embryos (Onodera et al. 2017). If the ICM is located near the hatching point, it is more likely to result in the separation into two or more groups of ICM (Fig. 1G).
However, animal studies have revealed that the hatching process in vivo differs significantly from that in vitro (Montag et al. 2000; Seshagiri et al. 2009; Vajta et al. 2010). In vivo, hatching occurs rapidly with the assistance of lytic factors like proteases present in the uterus. The ZP undergoes global solubilization and complete lysis without expansion and collapse in vivo. However 8-shaped hatching is more likely to happen in vitro when a small hatching point or gap is created in the ZP by assisted hatching (Yan et al. 2015).
Monochorionic monoamniotic monozygotic twins
In the late human blastocyst, the ICM undergoes differentiation into two distinct cell types: the hypoblast, which gives rise to the yolk sac, and the epiblast, which develops into the embryonic body and the amnion, a membrane that directly surrounds the human fetus (Molè et al. 2020; Rossant and Tam 2022). In instances where the epiblast of the ICM is not completely separated into two parts but remains partially connected, the amnion of twins within a single chorion can also merge, leading to monochorionic monoamniotic twins (Fig. 1B). Further investigation is required to identify factors that regulate the spacing between separated ICM clusters during blastocyst cavitation, thereby influencing the number of amnions.
A similar phenomenon can be observed in the zebrafish MZ twin mutant known as janus, where the spacing of blastomeres also determines the outcome of the twin phenotype (Abdelilah et al. 1994; Abdelilah and Driever 1997). In the Zebrafish janus mutant, blastomeres divide into two groups during the first four cleavages and ultimately attach to different sites on the embryonic yolk. If the distance between the separated blastomeres is too close, the blastoderm will partially fuse during development, resulting in a conjoined marginal zone (Abdelilah and Driever 1997). While the janus mutant can mimic certain aspects of the ICM separation process, the phenotype is unstable, and the mutated gene has not been identified.
However, according to the classic fission model of human monochorionic monoanionic MZ twins, it is believed that they arise from the division of the ICM subsequent to hatching, even after implantation, occurring after embryonic day 8 (Fig. 1B) (Hall 2003; Kaufman 2004; McNamara et al. 2016). It is unclear whether monochorionic monoamniotic MZ twins result from partial ICM splitting during blastocyst cavitation or after hatching. While this type of MZ twins is uncommon for twin pregnancies, accounting for only 1–2% of liveborn MZ twins (Hall 2003), it poses a significant risk of giving rise to conjoined twins if the two clusters of ICMs are not fully separated (Johnston 2001). Due to their low rate of occurrence and late splitting, it is difficult to observe their development in vitro through live imaging. Additionally, the lack of a suitable animal model makes it challenging to study the mechanism of monochorionic monoanionic MZ twinning. Resolving this mystery could significantly contribute to preventing the occurrence of conjoined twins, a situation that poses extremely high health risks to the progeny associated with a substantial financial burden on their families.
Cellular mechanism of dichorionic monozygotic twinning
In natural pregnancies, dichorionic twins are commonly but not necessarily correctly assumed to be dizygotic, originating from two separately fertilized oocytes. Their zygosity can only be confirmed through genetic testing, like DNA fingerprinting (McLaren et al. 1995) and short tandem repeat profiling (Brouillet et al. 2022; Semrl et al. 2023). As a result, studying the mechanisms of dichorionic MZ twinning has been challenging until the reports of numerous ART cases where single blastocyst transfers resulted in dichorionic twin pregnancies (Klein et al. 2005; Kyono 2013; Sundaram et al. 2018; Konno et al. 2020; Li et al. 2020; Neumann et al. 2020; Dirican and Olgan 2021; Brouillet et al. 2022; Semrl et al. 2023). Most recently, there has even been the first reported case of a dichorionic diamniotic triplet pregnancy after a single blastocyst transfer (Cara et al. 2023). However, these dichorionic twins might have been dizygotic even after single embryo transfer, with one of the twins developing from the transplanted embryo of ART, while the other one developed through natural conception following ovulation (van der Hoorn et al. 2011; Osianlis et al. 2014; Takehara et al. 2014). To eliminate the possibility that these dichorionic twins are dizygotic, some studies have conducted genetic testing to confirm their monozygosity (Krishnan et al. 2008; Brouillet et al. 2022; Semrl et al. 2023).
Yet, the main point of contention in dichorionic twinning is the timing of the splitting process. Initially, it was hypothesized that the separation occurs at an early stage before embryonic day 3 to generate two separate blastocysts, each forming an individual MZ twin with its own chorion and amnion (Fig. 2A) (Corner 1922). Later it was proposed that the splitting can occur even as early as right after the first cleavage at two-cell stage, between the two blastomeres (Herranz 2015). However, the recent ART cases suggest that the splitting of dichorionic MZ twins may occur at the blastocyst stage or later, which could represent an alternative mechanism compared to the existing model (Klein et al. 2005; Kyono 2013; Sundaram et al. 2018; Konno et al. 2020; Li et al. 2020; Neumann et al. 2020; Dirican and Olgan 2021; Brouillet et al. 2022; Semrl et al. 2023). In this section, we will first discuss the classic scenario of early embryo splitting before the morula stage, and then explore the mechanisms behind a single blastocyst transfer leading to dichorionic MZ twins, based on ART cases and mouse experiments.
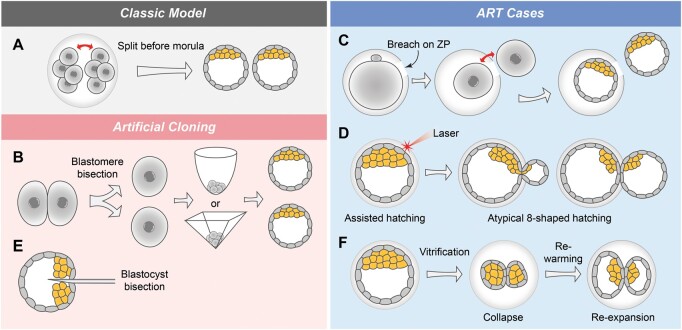
Figure 2.
Formation of dichorionic monozygotic (MZ) twins. (A) The classic model of dichorionic MZ twin formation proposed that it occurs when blastomeres split before the morula stage. (B) The divided blastomeres of zona pellucida (ZP)-free mouse embryos at the two-cell stage can be separately cultured in U-shaped or V-shaped bottom wells, eventually developing into small blastocysts. (C) In an ART case, one of the blastomere emerged from the ZP through a breach at the two-cell stage, with each blastomere forming an individual blastocyst. (D) Dichorionic MZ twins can result from the separation of the ICM and trophectoderm during atypical 8-shaped hatching, forming two individual small blastocysts. (E) In certain species such as sheep, cattle, goat, and pig, embryos can be replicated by splitting a blastocyst into two halves using a sharp needle, each containing a similar number of ICM and TE cells. (F) Blastocyst separation was observed in an ART case in a vitrified-warmed cycle, leading to a dichorionic MZ twin pregnancy.
Can early splitting lead to dichorionic twins under physiological conditions? Lessons from artificial cloning
Blastomere biopsy of human embryos before compaction allows each group of blastomeres to develop into individual blastocysts (Illmensee et al. 2010; Noli et al. 2016), resembling the classic model of dichorionic MZ twins. However, the occurrence of this process under physiological conditions without manual intervention remains controversial.
Generation of MZ twins through blastomere separation was first performed in sheep and cow embryos (Willadsen 1980, 1989; Willadsen and Polge 1981). In mouse embryos, recent findings suggest that cell fate has diverged between the cells as early as the two-cell stage, with one blastomere exhibiting stronger totipotency than the other (Papaioannou et al. 1989; Casser et al. 2017; Hupalowska et al. 2018; Wang et al. 2018; Jin et al. 2022). However, in some cases, it is still possible to obtain artificially generated twin blastocysts and individuals through bisecting the blastomeres at the two-cell stage (Fig. 2B) (O'Brien et al. 1984; Motosugi et al. 2005; Roberts et al. 2011; Morris et al. 2012; Hisaki et al. 2014; Casser et al. 2017, 2019; Krawczyk et al. 2021). These artificial cloning processes could provide us with a valuable indication of the feasibility of the early splitting theory of dichorionic MZ twins.
Strict conditions are required to successfully generate MZ twin embryos in mice through blastomere separation. Firstly, the embryo needs to be released from the ZP by either creating a slit in the ZP or by completely removing the ZP with Tyrode's solution. Then, the sister blastomeres are to be separated using physical force like pipetting or a chemical agent like Trypsin. Next, to prevent conjoining, the ZP-free half embryos are to be cultured in separated spaces to block any junction between them. Furthermore, to ensure the proper 3-dimensional aggregation of blastomeres into blastocysts without the presence of the ZP, the bisected blastomeres are to be cultured in a restricted environment such as U-shaped or V-shaped bottom wells (Fig. 2B) (Casser et al. 2017; Liu et al. 2021; Yu et al. 2021), or even placed back into empty ZPs (Illmensee et al. 2005; Motosugi et al. 2005; Tang et al. 2012).
Drawing from the evidence of mouse experiments (Motosugi et al. 2005; Tang et al. 2012; Casser et al. 2017), three key questions need to be addressed on how separated human blastocysts can be achieved from early splitting embryos without manual intervention and under physiological conditions as hypothesized by the classic theory.
What physical force causes the splitting of blastomeres before the morula stage?
A thin ZP or a breached ZP is associated with MZ twinning, where the embryo can be relieved from the restriction of the ZP and undergo splitting (Alikani et al. 1994). Recently, an ART case of a spontaneous early splitting human embryo was observed using time-lapse live imaging (Matorras et al. 2023). The ZP of the early splitting human embryo ruptured during oocyte manipulation (Fig. 2C). Following sperm microinjection and the first cleavage, one of the two blastomeres emerged from the ZP, while the other remained inside. The two separated blastomeres developed individually into blastocysts, having the potential to give rise to dichorionic MZ twins (Fig. 2C). However, to make this scenario happen under physiological conditions, the question of which forces or factors can cause the rupture of the ZP in vivo needs to be answered.
The early splitting of the embryo could result from multiple mechanisms. An interphase bridge, a microtubule cytoskeleton-dense structure connecting sister blastomeres during interphase within the preimplantation embryo (Zenker et al. 2017), might lead to the separation of cells when breaking down during cell division. Additionally, the repulsion and contact inhibition could potentially occur between two separate blastomeres, mediated by pathways such as Eph/ephrin signaling, which regulates cell–cell contact and repulsion to preserve cellular or tissue boundaries (Pasquale 2005; Klein 2012; Zhang et al. 2016). A decrease in calcium levels has also been hypothesized to increase the rate of MZ twins by regulating cell adhesion and inducing early embryo splitting (Steinman 2001a,b; Steinman and Valderrama 2001).
How do twin embryos prevent themselves from rejoining in a physiological environment?
Ensuring the separation of blastocysts is crucial to avoid the fusion of embryos, as human embryos can fuse in group cultures (Schiewe et al. 2015; Swain 2021). In some cases, the chorions of dizygotic twins can fuse into one (Peters et al. 2017). Similarly, mouse blastocysts can also be fused into one chimeric blastocyst with two clusters of ICMs (Tarkowski and Wojewodzka 1982). Zebrafish MZ twins maintain their separation before hatching by attaching to different sites of the yolk, a unique feature that mammalian embryos lack (Abdelilah et al. 1994; Abdelilah and Driever 1997). Thus, to support the early-splitting theory of dichorionic MZ twins under a physiological environment, factors or mechanisms which could keep the twin blastomeres apart until implantation occurs need to be further investigated. One possible scenario could involve a breached-ZP, with one twin embryo developing outside, and the other inside, the ZP (Fig. 2C) (Matorras et al. 2023). This could also potentially be attributed to the cilia-driven fluid flow in the oviduct (Huang and Choma 2015), creating a spatial gap between the two distinct embryos.
How do the twin blastomeres ensure proper aggregation to avoid blastomere dispersal without ZP?
Women carrying a mutation in a ZP gene (ZP1, ZP2, or ZP3) face challenges in conceiving naturally due to the risk of abnormal fertilization and improper preimplantation embryo aggregation (Huang et al. 2014; Chen et al. 2017; Zhou et al. 2019, 2022; Luo et al. 2020; Sun et al. 2021; Zeng et al. 2023). ARTs such as ICSI and in vitro culture can help address these issues. In most cases, to ensure an intact embryo and successful pregnancy, ZP-free human embryos are cultured in vitro until the blastocyst stage before transplantation (Ueno et al. 2014; Dai et al. 2019; Cao et al. 2020; Watson et al. 2021). The earliest successful time point for ZP-free embryo transfer is Day 3 when the embryos begin to compact, which can improve the rate of successful pregnancy (Mansour et al. 2000). To enhance the development rate of ZP-free human embryos, the Well-of-the-Well (WOW) system was applied in vitro where V-shaped small wells are created within a larger well to facilitate the proper compaction (Vajta et al. 2000, 2008). ZP-free human embryos can also be placed back into empty ZP (Illmensee et al. 2010) or an artificial gel to support the development of these embryos (Song et al. 2022).
However, under physiological conditions, if the embryo split before morula stage, ZP-free twin embryos are challenging to maintain in an intact state due to low cell adhesion (Fleming et al. 2001), and may experience difficulties in undergoing normal compaction and blastocyst formation. To address whether early-split, ZP-free twin embryos can achieve proper aggregation and successful embryo development in vivo, we need model systems mimicking the physiological conditions. Several experimental setups have been established to replicate physiological fluid flow in vitro (Juste-Lanas et al. 2023), which can simulate the oviduct environment and study the development of early ZP-free embryos.
Overall, to generate dichorionic MZ twins through early splitting of embryos in physiological condition, the embryo must satisfy several stringent criteria: they require a physical force to split and exit the ZP at an early stage, while developing intact without dispersing after splitting, and they must also avoid touching and fusing with each other. The occurrence of dichorionic MZ twinning through early splitting is potentially lower under physiological conditions (Gardner 2014). Further studies and supporting evidence from both ART cases and animal experiments are necessary to understand how dichorionic MZ twins happen under physiological conditions.
Mechanisms of dichorionic monozygotic twinning after single blastocyst transfer
Atypical hatching causes higher rates of dichorionic MZ twins in ART
In ART, several cases have been reported where atypical hatching has led to a dichorionic MZ twin pregnancy after single blastocyst transfer (Van Langendonckt et al. 2000; Behr and Milki 2003; Kyono 2013; Sundaram et al. 2018; Jundi et al. 2021). In some atypical cases of the 8-shaped hatching, the slit on the ZP is small, allowing only half of the ICM to emerge from the ZP, while the other half remains inside. When both, the ICM and the TE, divide into two halves due to the pressure from the small slit drilled by laser, it may result in the formation of two separated blastocysts, leading to MZ twins, each with their own chorion (Fig. 2D) (Van Langendonckt et al. 2000; Sundaram et al. 2018; Jundi et al. 2021). This concept bears resemblance to the method of artificial cloning used in animal husbandry for mammals such as sheep, cattle goats, and pigs, where the blastocyst is directly bisected into two halves using a sharp needle (Fig. 2E) (Willadsen and Godke 1984; Williams et al. 1984; Tsunoda et al. 1985; Nagashima et al. 1989; Széll and Hudson 1991; Noli et al. 2016).
Vitrified-warmed cycles can lead to dichorionic MZ twins
To date, there is only one reported case in which an embryo from a vitrified-warmed cycle was observed to separate into two blastocysts, leading to the birth of dichorionic MZ twins (Fig. 2F) (Shibuya and Kyono 2012). During the re-expansion of the blastocoel after embryo freezing and re-warming, some cells may remain intact, coincidentally segregating the ICM and TE into two small, separated blastocysts formed inside the ZP (Fig. 2F). While this is the only reported case with a clear phenotype of blastocyst segregation, there are also unexplained cases of dichorionic MZ twin pregnancies following the transfer of a single vitrified-warmed blastocyst, which might share the same underlying mechanism (Li et al. 2020; Semrl et al. 2023).
Limitations and direction for future studies
Current models of monozygotic twinning and their limitations
Although humans are not the only species that exhibits MZ twins, there is currently no perfect animal model available to study the mechanism of human MZ twinning, which hinders research in this area. In this section, we will summarize the species currently known for MZ twinning and explain their limitations for studying human MZ twinning. Furthermore, we will propose in vitro human embryo models that hold promise for studying human MZ twins in future research.
Zebrafish
Zebrafish is a widely used vertebrate model organism, leveraging its significant advantage of in vitro fertilization and growth, which allows for the comprehensive observation of the entire developmental process (Astell and Sieger 2020). However, it exhibits notable differences in embryo structure and developmental patterns compared to mammalian embryos (upper panel of Fig. 3A), posing challenges in its application for studying human twinning. A zebrafish MZ twin mutant, known as janus, displays two distinct clusters of blastomeres attached closely to a single yolk (lower panel of Fig. 3A) (Abdelilah et al. 1994). However, the mechanisms governing the splitting of blastomeres are not fully understood, and the gene responsible for this natural mutation has yet to be identified. The primary challenge is the instability of the phenotype, which is temperature-sensitive and inherited through a maternal recessive mode (Abdelilah and Driever 1997). Overall, the structural disparities of zebrafish embryos and intricate inheritance patterns of the mutant render it less than an ideal animal model for studying human MZ twinning.
Figure 3.
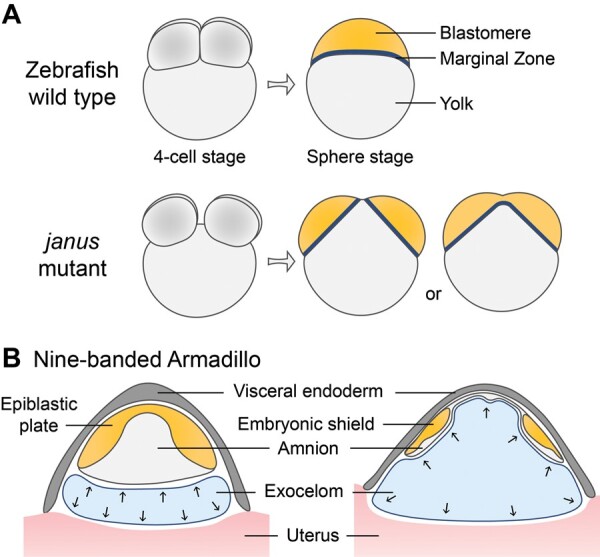
Animals that can produce monozygotic (MZ) twins or quadruplets. (A) The zebrafish janus mutant exhibits the separation of two groups of blastomeres prior to the eight-cell stage, resulting in the development of two distinct spheres (yellow) that ultimately lead to the formation of conjoined fish. If there is limited space between the two groups of cells, the blastomeres and marginal zones (dark blue) will merge during development. (B) To generate MZ quadruplets, zygotic splitting of nine-banded armadillo embryos happens after implantation. The substantial enlargement of the exocelom cavity (blue) functions as a physical force, causing the division of the embryonic shields that develop from the epiblastic plate (yellow), and effectively separating them into distinct spaces that remain unconnected.
Nine-banded armadillo
The nine-banded armadillo is the sole animal known to naturally and consistently give birth to identical quadruplets (Carter 2018). However, the timing of zygotic splitting occurs after implantation, which is markedly distinct from human twinning (Prodöhl et al. 1996; Enders 2002; Blickstein and Keith 2007). In the nine-banded armadillo, the embryo does not implant right after the formation of blastocyst. Instead, it experiences a delay that can span several months. After blastocyst implantation, the ICM gives rise to a single amnion and an epiblastic plate. Between the TE implantation site and the amnion, a distinctive cavity named the exocelom forms (Fig. 3B) (Enders 2002). Subsequently, the epiblastic plate undergoes differentiation into separated embryonic shields, each capable of developing into an individual. As the exocelom cavity expands significantly, it works as a physical force and ultimately splits the four embryonic shields to distinct locations, giving rise to identical quadruplets (Fig. 3B). This mechanism is unique to this specific species and does not offer significant insights into human MZ twinning. Additionally, the nine-banded armadillo is not commonly employed as a model organism used in research laboratories and is thus not readily accessible for embryonic research purposes.
Mouse
While mice are the most commonly used mammalian model in current scientific studies, fundamental differences confine their resemblance with human embryos for MZ twin research. The natural occurrence of mouse MZ twinning under physiological conditions is exceedingly rare (McLaren et al. 1995). One significant issue is that mice are not a single-birth animals, making it challenging to identify which two embryos originated from one oocyte without genetic testing. Additionally, murine MZ twin embryos might thus face disadvantages by having fewer cells than the neighboring embryos that originate entirely from a single fertilized oocyte, which could result in their loss during natural competition. Therefore, it is less likely to obtain MZ twins in mice. Moreover, the development of mouse embryos differs significantly from that of human embryos in various aspects, such as timing of zygotic genome activation, compaction, implantation, gastrulation, and more (Molè et al. 2020; Bissiere et al. 2023). Especially, human embryos implant from the polar TE attaching to the ICM, while mouse embryos implant from the mural TE which is opposite to the ICM (Muter et al. 2023). Due to these differences, the development of MZ twins cannot be fully replicated or accurately mimicked using mouse embryos, which possess their unique characteristics. It is only useful in situations where insights from human ART cases were obtained and potential mechanisms have been evaluated and validated in mouse embryos at specific preimplantation stages, such as atypical 8-shaped hatching and ICM separation. Thus, studying MZ twinning in human embryos during ART procedures with increased MZ twin birth rates still offers the most direct and effective approach to address the fundamental questions raised in this review.
In vitro models of human embryogenesis
In ART therapy, human embryos can only be cultured in vitro to blastocyst stage before they are implanted into the uterus, which is a main restriction in research to observe the human embryo beyond the blastocyst stage. The extended cultivation of human embryos in vitro for scientific research is strictly limited due to ethical considerations. As a result, we are lacking information about whether the ICM can divide after hatching or even after implantation to generate monochorionic MZ twins. Furthermore, there have been numerous inexplicable ART cases where a single blastocyst transfer leads to MZ twins (Konno et al. 2020; Li et al. 2020; Neumann et al. 2020; Semrl et al. 2023) or even MZ triplets (Faraj et al. 2008; Lee et al. 2008; Dessolle et al. 2010; Gurunath et al. 2015; Saravelos et al. 2016). Recent advancements in creating in vitro systems to model early human embryogenesis using induced pluripotent stem cells (iPSCs) or isolated human embryonic stem cells have shown promising results, with the development of blastocyst-like structures called blastoids (Liu et al. 2021; Yu et al. 2021) and even post-implantation human embryoids (Pedroza et al. 2023; Weatherbee et al. 2023). These models may provide unprecedent opportunities to investigate whether the ICM and TE can still separate after hatching or even after implantation.
In fact, double ICMs within a single blastocoel have been observed in some twin blastoids, a valuable model for studying human MZ twins in vitro, by increasing the cell number and treating the twin blastoids with lysophosphatidic acid. The ICMs separated during cavity expansion, with each ICM containing both epiblast and hypoblast cells, mimicking the phenotype of monochorionic MZ twins. This in vitro model system may be further used to investigate the mechanisms underlying ICM separation, the control of ICM spacing, and post-implantation development of MZ twins (Luijkx et al. 2024).
Searching for ‘twin genes’
Currently, our understanding of the mechanism of MZ twins is primarily centered around the cellular level, and the genetic mechanisms that drive this process still require further investigation and discovery. There is a contention that the elevated rate of MZ twins in ART is not attributed to the technology itself, but rather to the genetic background of the embryos (Sobek et al. 2015).
Scientists believe that MZ twins are controlled by genetic regulation for two reasons. Firstly, unlike dizygotic twins, the occurrence of MZ twins within the population is relatively consistent across different regions, with an approximate rate of 1 in 250 pregnancies (McNamara et al. 2016). Secondly, there have been reports of familial cases of MZ twins spanning up to four generations (Steinman 2003; Hamamy et al. 2004; Cyranoski 2009; Machin 2009a; Liu et al. 2018b). Numerous studies have been conducted by scientists to uncover the genetic mechanisms underlying the formation of MZ twins (Lichtenstein et al. 1998; Cyranoski 2009; Liu et al. 2018b). MZ twinning is inherited in an autosomal dominant manner, and genes related to cell adhesion are consistently on the list of suspects responsible for MZ twinning (Bamforth et al. 2003; Machin 2009b). Through whole genome sequencing of a four-generation MZ twin pedigree, enrichment of single-nucleotide variants and copy number variants were observed within the epithelial adherens junction signaling pathway, GTPase family-mediated pathways, and tight junction signaling pathways (Liu et al. 2018b). Mutated genes may reduce the adhesion of the ICM, resulting in the division of the ICM during the cavitation of the blastocoel. Despite significant efforts, specific genes directly responsible for human MZ twins have not yet been identified. The genetic mechanism behind MZ twins is challenging to uncover because it may not be linked to the mutation of a single gene, and even if there is a mutation, its effect may not be fully penetrant.
In addition to the genetic mechanism, epigenetic hallmarks for MZ twins have recently been identified (van Dongen et al. 2021, 2024). Differentially methylated positions between MZ and dizygotic twins remain consistently present in their somatic cells, gauging a new way of identifying individuals as MZ twins. Genes related to cell-adhesion pathways showed significant enrichment among the genes nearest to the differentially methylated positions.
The discovery of ‘twin genes’ may allow further insights into the cellular mechanism of MZ twins at both the genetic and the epigenetic levels, and into twin rates associated with regional origins or pedigrees.
Implications for reducing the monozygotic twinning rate in ART therapy
As ART advances and achieves higher success rates, the practice of single embryo transfer has become prevalent to mitigate the occurrence of multiple pregnancies (Pinborg 2005; Reimundo et al. 2021; De Neubourg et al. 2022; Fouks and Yogev 2022). However, MZ twinning still remains possible following single embryo transfer. Based on the cellular mechanisms analyzed in our review through reported ART cases and animal studies, the following measures should be considered to minimize the occurrence of MZ twins during IVF process. It is essential to thoroughly monitor the development of ART embryos and confirm the phenotype of blastocysts prior to transplantation, using cutting-edge technologies such as high-resolution time-lapse live imaging (Zenker et al. 2017, 2018; Sutherland et al. 2019; Hawdon et al. 2023; Matorras et al. 2023). To reduce the occurrence of monochorionic MZ twinning, blastocysts displaying a loosely connected ICM or those divided into multiple groups should be avoided. To decrease the incidence of dichorionic MZ twin pregnancies, embryos displaying atypical 8-shaped hatching or divided blastocysts should not be the primary choice for transplantation. When performing assisted hatching, it is important to create the artificial hatching site at a distance from the ICM to prevent the ICM splitting during 8-shaped hatching. Furthermore, a technique more akin to the natural degradation of the ZP rather than creating a single small hole may be beneficial. Implementing these measures can help to reduce the occurrence of MZ twins in ART to some extent.
Conclusion
In summary, our knowledge on the cellular mechanisms of monochorionic MZ twins is advancing, and involves loose ICM splitting during multi-point blastocoel expansion, resulting in separate ICM clusters within the blastocyst. On the other hand, the natural occurrence of dichorionic MZ twins remains poorly understood and highly controversial. In ART, atypical 8-shaped hatching and vitrified-warmed cycles have been associated with blastocyst separation and the formation of dichorionic MZ twins. However, the mechanisms occurring under natural physiological conditions appear to be distinct and unclear. To gain further insights, the MZ twinning model requires continuous examination and potential modifications, particularly with the accumulation of future reported cases from ART procedures, animal experiments, and human models using iPSC-derived blastoids, gastruloids, and other embryonic organoids. With updates from MZ twinning models, the embryo transfer strategy in ART should also be adjusted to lower the rate of MZ twins. Additionally, exploring the genetic and epigenetic mechanisms involved will take us another step closer to unraveling the mystery of MZ twins.
Acknowledgements
We greatly appreciate Dr Marivic Tan’s thorough proofreading of the manuscript.
Contributor Information
Hongbin Jin, Australian Regenerative Medicine Institute, Monash University, Clayton, VIC, Australia.
Yang Han, Division of Cellular and Developmental Biology, Molecular and Cell Biology Department, University of California, Berkeley, CA, USA.
Jennifer Zenker, Australian Regenerative Medicine Institute, Monash University, Clayton, VIC, Australia.
Data availability
No new data were generated or analyzed in support of this review.
Authors’ roles
All authors engaged in an in-depth discussion about the topic and thoroughly reviewed the text. H.J. wrote the first draft of the manuscript.
Funding
This work was supported by the National Health and Medical Research Council (NHMRC) Ideas Grant APP2002507 and Investigator Grant APP2009409 to J.Z. J.Z. was also supported by the Sylvia & Charles Viertel Senior Medical Fellowship and the Canadian Institute for Advanced Research (CIFAR) Azrieli Scholarship. The Australian Regenerative Medicine Institute is supported by grants from the State Government of Victoria and the Australian Government.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Abdelilah S, Driever W.. Pattern formation in janus-mutant zebrafish embryos. Dev Biol 1997;184:70–84. [DOI] [PubMed] [Google Scholar]
- Abdelilah S, Solnica-Krezel L, Stainier DY, Driever W.. Implications for dorsoventral axis determination from the zebrafish mutation janus. Nature 1994;370:468–471. [DOI] [PubMed] [Google Scholar]
- Alikani M, Cekleniak NA, Walters E, Cohen J.. Monozygotic twinning following assisted conception: an analysis of 81 consecutive cases. Hum Reprod 2003;18:1937–1943. [DOI] [PubMed] [Google Scholar]
- Alikani M, Noyes N, Cohen J, Rosenwaks Z.. Monozygotic twinning in the human is associated with the zona pellucida architecture. Hum Reprod 1994;9:1318–1321. [DOI] [PubMed] [Google Scholar]
- Astell KR, Sieger D.. Zebrafish in vivo models of cancer and metastasis. Cold Spring Harb Perspect Med 2020;10:a037077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston KI, Peterson CM, Carrell DT.. Monozygotic twinning associated with assisted reproductive technologies: a review. Reproduction 2008;136:377–386. [DOI] [PubMed] [Google Scholar]
- Bamforth F, Brown L, Senz J, Huntsman D.. Mechanisms of monozygotic (MZ) twinning: a possible role for the cell adhesion molecule, E-cadherin. Am J Med Genet A 2003;120a:59–62. [DOI] [PubMed] [Google Scholar]
- Behr B, Fisch JD, Racowsky C, Miller K, Pool TB, Milki AA.. Blastocyst-ET and monozygotic twinning. J Assist Reprod Genet 2000;17:349–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr B, Milki AA.. Visualization of atypical hatching of a human blastocyst in vitro forming two identical embryos. Fertil Steril 2003;80:1502–1503. [DOI] [PubMed] [Google Scholar]
- Bissiere S, Hernandez B, Rubio C, Simón C, Plachta N.. Updates on preimplantation embryo research. Fertil Steril 2023;120:467–472. [DOI] [PubMed] [Google Scholar]
- Blickstein I, Keith LG.. On the possible cause of monozygotic twinning: lessons from the 9-banded armadillo and from assisted reproduction. Twin Res Hum Genet 2007;10:394–399. [DOI] [PubMed] [Google Scholar]
- Brouillet S, Mereuze S, Ranisavljevic N, Chauveau C, Hamamah S, Cattin J, Verebi C, Cabrol C, Ishmukhametova A, Girardet A. et al. Molecular characterization of a rare case of monozygotic dichorionic diamniotic twin pregnancy after single blastocyst transfer in preimplantation genetic testing (PGT). Int J Mol Sci 2022;23:10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnelli A, Dallagiovanna C, Reschini M, Paffoni A, Fedele L, Somigliana E.. Risk factors for monozygotic twinning after in vitro fertilization: a systematic review and meta-analysis. Fertil Steril 2019;111:302–317. [DOI] [PubMed] [Google Scholar]
- Cao Q, Zhao C, Zhang X, Zhang H, Lu Q, Wang C, Hu Y, Ling X, Zhang J, Huo R.. Heterozygous mutations in ZP1 and ZP3 cause formation disorder of ZP and female infertility in human. J Cell Mol Med 2020;24:8557–8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cara S, Bafaro MG, Cattoli M, Coticchio G, Di Paola R, Borini A.. First case of dichorionic diamniotic triplet pregnancy after single blastocyst transfer. J Assist Reprod Genet 2023;41:437–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AM. Classics revisited: Miguel Fernández on germ layer inversion and specific polyembryony in armadillos. Placenta 2018;61:55–60. [DOI] [PubMed] [Google Scholar]
- Casser E, Israel S, Boiani M.. Multiplying embryos: experimental monozygotic polyembryony in mammals and its uses. Int J Dev Biol 2019;63:143–155. [DOI] [PubMed] [Google Scholar]
- Casser E, Israel S, Witten A, Schulte K, Schlatt S, Nordhoff V, Boiani M.. Totipotency segregates between the sister blastomeres of two-cell stage mouse embryos. Sci Rep 2017;7:8299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Bian Y, Liu X, Zhao S, Wu K, Yan L, Li M, Yang Z, Liu H, Zhao H. et al. A recurrent missense mutation in ZP3 causes empty follicle syndrome and female infertility. Am J Hum Genet 2017;101:459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida S. Monozygous double inner cell masses in mouse blastocysts following fertilization in vitro and in vivo. J In Vitro Fert Embryo Transf 1990;7:177–179. [DOI] [PubMed] [Google Scholar]
- Cohen J. Assisted hatching of human embryos. J In Vitro Fert Embryo Transf 1991;8:179–190. [DOI] [PubMed] [Google Scholar]
- Corner GW. The morphological theory of monochorionic twins as illustrated by a series of supposed early twin embryos of the pig. Bull Johns Hopkins Hosp 1922;33:389–392. [Google Scholar]
- Corner GW. The observed embryology of human single-ovum twins and other multiple births. Am J Obstet Gynecol 1955;70:933–951. [DOI] [PubMed] [Google Scholar]
- Corsello G, Piro E.. The world of twins: an update. J Matern Fetal Neonatal Med 2010;23(Suppl 3):59–62. [DOI] [PubMed] [Google Scholar]
- Cyranoski D. Developmental biology: two by two. Nature 2009;458:826–829. [DOI] [PubMed] [Google Scholar]
- Dai C, Hu L, Gong F, Tan Y, Cai S, Zhang S, Dai J, Lu C, Chen J, Chen Y. et al. ZP2 pathogenic variants cause in vitro fertilization failure and female infertility. Genet Med 2019;21:431–440. [DOI] [PubMed] [Google Scholar]
- De Neubourg D, Dancet EAF, Pinborg A.. Single-embryo transfer implies quality of care in reproductive medicine. Reprod Biomed Online 2022;45:899–905. [DOI] [PubMed] [Google Scholar]
- De Sutter DP. Single embryo transfer (set) not only leads to a reduction in twinning rates after IVF/ICSI, but also improves obstetrical and perinatal outcome of singletons. Verh K Acad Geneeskd Belg 2006;68:319–327. [PubMed] [Google Scholar]
- Dessolle L, Allaoua D, Fréour T, Le Vaillant C, Philippe HJ, Jean M, Barrière P.. Monozygotic triplet pregnancies after single blastocyst transfer: two cases and literature review. Reprod Biomed Online 2010;21:283–289. [DOI] [PubMed] [Google Scholar]
- Ding J, Yin T, Zhang Y, Zhou D, Yang J.. The effect of blastocyst transfer on newborn sex ratio and monozygotic twinning rate: an updated systematic review and meta-analysis. Reprod Biomed Online 2018;37:292–303. [DOI] [PubMed] [Google Scholar]
- Dirican EK, Olgan S.. On the origin of zygosity and chorionicity in twinning: evidence from human in vitro fertilization. J Assist Reprod Genet 2021;38:2809–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumortier JG, Le Verge-Serandour M, Tortorelli AF, Mielke A, de Plater L, Turlier H, Maître JL.. Hydraulic fracturing and active coarsening position the lumen of the mouse blastocyst. Science 2019;365:465–468. [DOI] [PubMed] [Google Scholar]
- Eliasen T, Gabrielsen A, Bay B, Iversen L, Knudsen U.. Monochorionic twins after single blastocyst transfer: retrospective cohort and blinded time lapse annotation analysis. Reprod Biomed Online 2021;43:62–65. [DOI] [PubMed] [Google Scholar]
- Elizur SE, Levron J, Shrim A, Sivan E, Dor J, Shulman A.. Monozygotic twinning is not associated with zona pellucida micromanipulation procedures but increases with high-order multiple pregnancies. Fertil Steril 2004;82:500–501. [DOI] [PubMed] [Google Scholar]
- Enders AC. Implantation in the nine-banded armadillo: how does a single blastocyst form four embryos? Placenta 2002;23:71–85. [DOI] [PubMed] [Google Scholar]
- Faraj R, Evbuomwan I, Sturgiss S, Aird I.. Monozygotic triplet pregnancy following egg donation and transfer of single frozen-thawed embryo. Fertil Steril 2008;89:1260.e9–1260.e12. [DOI] [PubMed] [Google Scholar]
- Fleming TP, Sheth B, Fesenko I.. Cell adhesion in the preimplantation mammalian embryo and its role in trophectoderm differentiation and blastocyst morphogenesis. Front Biosci 2001;6:D1000–D1007. [DOI] [PubMed] [Google Scholar]
- Fouks Y, Yogev Y.. Twinning in ART: single embryo transfer policy. Best Pract Res Clin Obstet Gynaecol 2022;84:88–95. [DOI] [PubMed] [Google Scholar]
- Gardner RL. The timing of monozygotic twinning: a pro-life challenge to conventional scientific wisdom. Reprod Biomed Online 2014;28:276–278. [DOI] [PubMed] [Google Scholar]
- Gu YF, Zhou QW, Zhang SP, Lu CF, Gong F, Tan YQ, Lu GX, Lin G.. Inner cell mass incarceration in 8-shaped blastocysts does not increase monozygotic twinning in preimplantation genetic diagnosis and screening patients. PLoS One 2018;13:e0190776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunath S, Makam A, Vinekar S, Biliangady RH.. Monochorionic triamniotic triplets following conventional in vitro fertilization and blastocyst transfer. J Hum Reprod Sci 2015;8:54–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JG. Twinning. Lancet 2003;362:735–743. [DOI] [PubMed] [Google Scholar]
- Hamamy HA, Ajlouni HK, Ajlouni KM.. Familial monozygotic twinning: report of an extended multi-generation family. Twin Res 2004;7:219–222. [DOI] [PubMed] [Google Scholar]
- Hammadeh ME, Fischer-Hammadeh C, Ali KR.. Assisted hatching in assisted reproduction: a state of the art. J Assist Reprod Genet 2011;28:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawdon A, Geoghegan ND, Mohenska M, Elsenhans A, Ferguson C, Polo JM, Parton RG, Zenker J.. Apicobasal RNA asymmetries regulate cell fate in the early mouse embryo. Nat Commun 2023;14:2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz G. The timing of monozygotic twinning: a criticism of the common model. Zygote 2015;23:27–40. [DOI] [PubMed] [Google Scholar]
- Hisaki T, Kawai I, Sugiura K, Naito K, Kano K.. Regulation of embryonic size in early mouse development in vitro culture system. Zygote 2014;22:340–347. [DOI] [PubMed] [Google Scholar]
- Hoekstra C, Zhao ZZ, Lambalk CB, Willemsen G, Martin NG, Boomsma DI, Montgomery GW.. Dizygotic twinning. Hum Reprod Update 2008;14:37–47. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Gonda MA.. Monozygotic twin formation in mouse embryos in vitro. Science 1980;209:605–606. [DOI] [PubMed] [Google Scholar]
- Huang BK, Choma MA.. Microscale imaging of cilia-driven fluid flow. Cell Mol Life Sci 2015;72:1095–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HL, Lv C, Zhao YC, Li W, He XM, Li P, Sha AG, Tian X, Papasian CJ, Deng HW. et al. Mutant ZP1 in familial infertility. N Engl J Med 2014;370:1220–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupalowska A, Jedrusik A, Zhu M, Bedford MT, Glover DM, Zernicka-Goetz M.. CARM1 and paraspeckles regulate pre-implantation mouse embryo development. Cell 2018;175:1902–1916.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hviid KVR, Malchau SS, Pinborg A, Nielsen HS.. Determinants of monozygotic twinning in ART: a systematic review and a meta-analysis. Hum Reprod Update 2018;24:468–483. [DOI] [PubMed] [Google Scholar]
- Illmensee K, Kaskar K, Zavos PM.. Efficient blastomere biopsy for mouse embryo splitting for future applications in human assisted reproduction. Reprod Biomed Online 2005;11:716–725. [DOI] [PubMed] [Google Scholar]
- Illmensee K, Levanduski M, Vidali A, Husami N, Goudas VT.. Human embryo twinning with applications in reproductive medicine. Fertil Steril 2010;93:423–427. [DOI] [PubMed] [Google Scholar]
- Imaizumi Y. A comparative study of zygotic twinning and triplet rates in eight countries, 1972-1999. J Biosoc Sci 2003;35:287–302. [DOI] [PubMed] [Google Scholar]
- Jain JK, Boostanfar R, Slater CC, Francis MM, Paulson RJ.. Monozygotic twins and triplets in association with blastocyst transfer. J Assist Reprod Genet 2004;21:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Han Y, Wang H, Li JXH, Shen W, Zhang L, Chen L, Jia S, Yuan P, Chen H. et al. The second polar body contributes to the fate asymmetry in the mouse embryo. Natl Sci Rev 2022;9:nwac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston I. Conjoined twins. Lancet 2001;357:149. [DOI] [PubMed] [Google Scholar]
- Jundi SI, Pereira NCA, Merighi TM, Santos JFD, Yadid IM, Coslovsky M, Criscuolo TS, Iaa P.. Monozygotic dichorionic-diamniotic twin pregnancy after single embryo transfer at blastocyst stage: a case report. JBRA Assist Reprod 2021;25:168–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juste-Lanas Y, Hervas-Raluy S, García-Aznar JM, González-Loyola A.. Fluid flow to mimic organ function in 3D in vitro models. APL Bioeng 2023;7:031501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MH. The embryology of conjoined twins. Childs Nerv Syst 2004;20:508–525. [DOI] [PubMed] [Google Scholar]
- Kawachiya S, Bodri D, Shimada N, Kato K, Takehara Y, Kato O.. Blastocyst culture is associated with an elevated incidence of monozygotic twinning after single embryo transfer. Fertil Steril 2011;95:2140–2142. [DOI] [PubMed] [Google Scholar]
- Klein J, Grunfeld L, Duke M, Mukherjee T, Sandler B, Copperman AB.. Challenging traditional dogma: a report of two cases of late monozygotic splitting resulting in dichorionic twinning. Fertil Steril 2005;84:S238–S239. [Google Scholar]
- Klein R. Eph/ephrin signalling during development. Development 2012;139:4105–4109. [DOI] [PubMed] [Google Scholar]
- Knopman J, Krey LC, Lee J, Fino ME, Novetsky AP, Noyes N.. Monozygotic twinning: an eight-year experience at a large IVF center. Fertil Steril 2010;94:502–510. [DOI] [PubMed] [Google Scholar]
- Konno H, Murakoshi T, Miura K, Masuzaki H.. The incidence of dichorionic diamniotic twin pregnancy after single blastocyst embryo transfer and zygosity: 8 years of single-center experience. Twin Res Hum Genet 2020;23:51–54. [DOI] [PubMed] [Google Scholar]
- Krawczyk K, Kosyl E, Częścik-Łysyszyn K, Wyszomirski T, Maleszewski M.. Developmental capacity is unevenly distributed among single blastomeres of 2-cell and 4-cell stage mouse embryos. Sci Rep 2021;11:21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan N, Buchanan PM, Dzebisashvili N, Xiao H, Schnitzler MA, Brennan DC.. Monozygotic transplantation: concerns and opportunities. Am J Transplant 2008;8:2343–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyono K. The precise timing of embryo splitting for monozygotic dichorionic diamniotic twins: when does embryo splitting for monozygotic dichorionic diamniotic twins occur? Evidence for splitting at the morula/blastocyst stage from studies of in vitro fertilization. Twin Res Hum Genet 2013;16:827–832. [DOI] [PubMed] [Google Scholar]
- Landeira D, Bagci H, Malinowski AR, Brown KE, Soza-Ried J, Feytout A, Webster Z, Ndjetehe E, Cantone I, Asenjo HG. et al. Jarid2 coordinates nanog expression and PCP/Wnt signaling required for efficient ESC differentiation and early embryo development. Cell Rep 2015;12:573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SF, Chapman M, Bowyer L.. Monozygotic triplets after single blastocyst transfer: case report and literature review. Aust N Z J Obstet Gynaecol 2008;48:583–586. [DOI] [PubMed] [Google Scholar]
- Li H, Shen T, Sun X.. Monozygotic dichorionic-diamniotic pregnancies following single frozen-thawed blastocyst transfer: a retrospective case series. BMC Pregnancy Childbirth 2020;20:768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Källén B, Köster M.. No paternal effect on monozygotic twinning in the Swedish Twin Registry. Twin Res 1998;1:212–215. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu J, Chen S, Kang X, Du H, Li L.. Elevated incidence of monozygotic twinning is associated with extended embryo culture, but not with zona pellucida manipulation or freeze-thaw procedure. Fertil Steril 2018a;109:1044–1050. [DOI] [PubMed] [Google Scholar]
- Liu S, Hong Y, Cui K, Guan J, Han L, Chen W, Xu Z, Gong K, Ou Y, Zeng C. et al. Four-generation pedigree of monozygotic female twins reveals genetic factors in twinning process by whole-genome sequencing. Twin Res Hum Genet 2018b;21:361–368. [DOI] [PubMed] [Google Scholar]
- Liu X, Tan JP, Schröder J, Aberkane A, Ouyang JF, Mohenska M, Lim SM, Sun YBY, Chen J, Sun G. et al. Modelling human blastocysts by reprogramming fibroblasts into iBlastoids. Nature 2021;591:627–632. [DOI] [PubMed] [Google Scholar]
- Luijkx DG, Ak A, Guo G, van Blitterswijk CA, Giselbrecht S, Vrij EJ.. Monochorionic twinning in bioengineered human embryo models. Adv Mater 2024;36:e2313306. [DOI] [PubMed] [Google Scholar]
- Luke B, Brown MB, Wantman E, Stern JE.. Factors associated with monozygosity in assisted reproductive technology pregnancies and the risk of recurrence using linked cycles. Fertil Steril 2014;101:683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Zhu L, Liu Z, Yang X, Xi Q, Li Z, Duan J, Jin L, Zhang X.. Novel mutations in ZP1 and ZP2 cause primary infertility due to empty follicle syndrome and abnormal zona pellucida. J Assist Reprod Genet 2020;37:2853–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machin G. Familial monozygotic twinning: a report of seven pedigrees. Am J Med Genet C Semin Med Genet 2009a;151c:152–154. [DOI] [PubMed] [Google Scholar]
- Machin G. Non-identical monozygotic twins, intermediate twin types, zygosity testing, and the non-random nature of monozygotic twinning: a review. Am J Med Genet C Semin Med Genet 2009b;151c:110–127. [DOI] [PubMed] [Google Scholar]
- Mansour RT, Rhodes CA, Aboulghar MA, Serour GI, Kamal A.. Transfer of zona-free embryos improves outcome in poor prognosis patients: a prospective randomized controlled study. Hum Reprod 2000;15:1061–1064. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJ.. Three decades of twin births in the United States, 1980-2009. NCHS Data Brief 2012;(80):1–8. [PubMed] [Google Scholar]
- Mateizel I, Santos-Ribeiro S, Done E, Van Landuyt L, Van de Velde H, Tournaye H, Verheyen G.. Do ARTs affect the incidence of monozygotic twinning? Hum Reprod 2016;31:2435–2441. [DOI] [PubMed] [Google Scholar]
- Matorras R, Vendrell A, Ferrando M, Larreategui Z.. Early spontaneous twinning recorded by time-lapse. Twin Res Hum Genet 2023;26:215–218. [DOI] [PubMed] [Google Scholar]
- McLaren A, Molland P, Signer E.. Does monozygotic twinning occur in mice? Genet Res 1995;66:195–202. [DOI] [PubMed] [Google Scholar]
- McNamara HC, Kane SC, Craig JM, Short RV, Umstad MP.. A review of the mechanisms and evidence for typical and atypical twinning. Am J Obstet Gynecol 2016;214:172–191. [DOI] [PubMed] [Google Scholar]
- Meintjes M, Guerami AR, Rodriguez JA, Crider-Pirkle SS, Madden JD.. Prospective identification of an in vitro-assisted monozygotic pregnancy based on a double-inner-cell-mass blastocyst. Fertil Steril 2001;76:S172–S173. [Google Scholar]
- Ménézo YJ, Sakkas D.. Monozygotic twinning: is it related to apoptosis in the embryo? Hum Reprod 2002;17:247–248. [DOI] [PubMed] [Google Scholar]
- Mio Y, Maeda K.. Time-lapse cinematography of dynamic changes occurring during in vitro development of human embryos. Am J Obstet Gynecol 2008;199:660.e1–660.e5. [DOI] [PubMed] [Google Scholar]
- Molè MA, Weberling A, Zernicka-Goetz M.. Comparative analysis of human and mouse development: from zygote to pre-gastrulation. Curr Top Dev Biol 2020;136:113–138. [DOI] [PubMed] [Google Scholar]
- Montag M, Koll B, Holmes P, van der V.. Significance of the number of embryonic cells and the state of the zona pellucida for hatching of mouse blastocysts in vitro versus in vivo. Biol Reprod 2000;62:1738–1744. [DOI] [PubMed] [Google Scholar]
- Morris SA, Guo Y, Zernicka-Goetz M.. Developmental plasticity is bound by pluripotency and the Fgf and Wnt signaling pathways. Cell Rep 2012;2:756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motosugi N, Bauer T, Polanski Z, Solter D, Hiiragi T.. Polarity of the mouse embryo is established at blastocyst and is not prepatterned. Genes Dev 2005;19:1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Hey K.. Twinning rates. Lancet 1997;349:1398–1399. [DOI] [PubMed] [Google Scholar]
- Muter J, Lynch VJ, McCoy RC, Brosens JJ.. Human embryo implantation. Development 2023;150:dev201507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima H, Kato Y, Ogawa S.. Microsurgical bisection of porcine morulae and blastocysts to produce monozygotic twin pregnancy. Gamete Res 1989;23:1–9. [DOI] [PubMed] [Google Scholar]
- Neumann K, Griesinger G, Weichert J.. Dichorionic-diamniotic twin pregnancy after elective single-embryo transfer of a blastocyst. Dtsch Arztebl Int 2020;117:716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noli L, Capalbo A, Ogilvie C, Khalaf Y, Ilic D.. Discordant growth of monozygotic twins starts at the blastocyst stage: a case study. Stem Cell Reports 2015;5:946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noli L, Ogilvie C, Khalaf Y, Ilic D.. Potential of human twin embryos generated by embryo splitting in assisted reproduction and research. Hum Reprod Update 2016;23:156–165. [DOI] [PubMed] [Google Scholar]
- O'Brien MJ, Critser ES, First NL.. Developmental potential of isolated blastomeres from early murine embryos. Theriogenology 1984;22:601–607. [DOI] [PubMed] [Google Scholar]
- Onodera Y, Takahashi K, Goto M, Anzai M, Ono N, Shirasawa H, Sato W, Miura H, Sato N, Sato A. et al. The location of “8”-shaped hatching influences inner cell mass formation in mouse blastocysts. PLoS One 2017;12:e0175150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osianlis T, Rombauts L, Gabbe M, Motteram C, Vollenhoven V.. Incidence and zygosity of twin births following transfers using a single fresh or frozen embryo. Hum Reprod 2014;29:1438–1443. [DOI] [PubMed] [Google Scholar]
- Otsuki J, Iwasaki T, Katada Y, Sato H, Furuhashi K, Tsuji Y, Matsumoto Y, Shiotani M.. Grade and looseness of the inner cell mass may lead to the development of monochorionic diamniotic twins. Fertil Steril 2016;106:640–644. [DOI] [PubMed] [Google Scholar]
- Papaioannou VE, Mkandawire J, Biggers JD.. Development and phenotypic variability of genetically identical half mouse embryos. Development 1989;106:817–827. [DOI] [PubMed] [Google Scholar]
- Papanikolaou EG, Fatemi H, Venetis C, Donoso P, Kolibianakis E, Tournaye H, Tarlatzis B, Devroey P.. Monozygotic twinning is not increased after single blastocyst transfer compared with single cleavage-stage embryo transfer. Fertil Steril 2010;93:592–597. [DOI] [PubMed] [Google Scholar]
- Parazzini F, Cipriani S, Bianchi S, Bulfoni C, Bortolus R, Somigliana E.. Risk of monozygotic twins after assisted reproduction: a population-based approach. Twin Res Hum Genet 2016;19:72–76. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol 2005;6:462–475. [DOI] [PubMed] [Google Scholar]
- Payne D, Okuda A, Wakatsuki Y, Takeshita C, Iwata K, Shimura T, Yumoto K, Ueno Y, Flaherty S, Mio Y.. Time-lapse recording identifies human blastocysts at risk of producing monozygotic twins. Hum Reprod 2007;22:i9–i11. [Google Scholar]
- Pedroza M, Gassaloglu SI, Dias N, Zhong L, Hou TJ, Kretzmer H, Smith ZD, Sozen B.. Self-patterning of human stem cells into post-implantation lineages. Nature 2023;622:574–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters HE, König TE, Verhoeven MO, Schats R, Mijatovic V, Ket JC, Lambalk CB.. Unusual twinning resulting in chimerism: a systematic review on monochorionic dizygotic twins. Twin Res Hum Genet 2017;20:161–168. [DOI] [PubMed] [Google Scholar]
- Pinborg A. IVF/ICSI twin pregnancies: risks and prevention. Hum Reprod Update 2005;11:575–593. [DOI] [PubMed] [Google Scholar]
- Prodöhl PA, Loughry WJ, McDonough CM, Nelson WS, Avise JC.. Molecular documentation of polyembryony and the micro-spatial dispersion of clonal sibships in the nine-banded armadillo, Dasypus novemcinctus. Proc Biol Sci 1996;263:1643–1649. [DOI] [PubMed] [Google Scholar]
- Reimundo P, Gutiérrez Romero JM, Rodríguez Pérez T, Veiga E.. Single-embryo transfer: a key strategy to reduce the risk for multiple pregnancy in assisted human reproduction. Adv Lab Med 2021;2:179–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RM, Katayama M, Magnuson SR, Falduto MT, Torres KE.. Transcript profiling of individual twin blastomeres derived by splitting two-cell stage murine embryos. Biol Reprod 2011;84:487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J, Tam PPL.. Early human embryonic development: blastocyst formation to gastrulation. Dev Cell 2022;57:152–165. [DOI] [PubMed] [Google Scholar]
- Saito H, Tsutsumi O, Noda Y, Ibuki Y, Hiroi M.. Do assisted reproductive technologies have effects on the demography of monozygotic twinning? Fertil Steril 2000;74:178–179. [DOI] [PubMed] [Google Scholar]
- Saravelos SH, Zhang T, Chung JP, Sun LM, Sun Y, Li TC, Chen ZJ.. Monochorionic quadramniotic and triamniotic pregnancies following single embryo transfers: two case reports and a review of the literature. J Assist Reprod Genet 2016;33:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiewe MC, Whitney JB, Anderson RE.. Potential risk of monochorionic dizygotic twin blastocyst formation associated with early laser zona dissection of group cultured embryos. Fertil Steril 2015;103:417–421. [DOI] [PubMed] [Google Scholar]
- Schimmel T, Cohen J, Saunders H, Alikani M.. Laser-assisted zona pellucida thinning does not facilitate hatching and may disrupt the in vitro hatching process: a morphokinetic study in the mouse. Hum Reprod 2014;29:2670–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciorio R, Meseguer M.. Focus on time-lapse analysis: blastocyst collapse and morphometric assessment as new features of embryo viability. Reprod Biomed Online 2021;43:821–832. [DOI] [PubMed] [Google Scholar]
- Semrl N, Barth M, Feigl S, Hochstätter R, Oreskovic I, Fluhr H, Klaritsch P, Speicher I, Kollmann M.. Birth of monozygotic dichorionic twins after a single blastocyst embryo transfer: a case report of genetic determination of zygosity. F S Rep 2023;4:231–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshagiri PB, Sen Roy S, Sireesha G, Rao RP.. Cellular and molecular regulation of mammalian blastocyst hatching. J Reprod Immunol 2009;83:79–84. [DOI] [PubMed] [Google Scholar]
- Sharara FI, Abdo G.. Incidence of monozygotic twins in blastocyst and cleavage stage assisted reproductive technology cycles. Fertil Steril 2010;93:642–645. [DOI] [PubMed] [Google Scholar]
- Shibuya Y, Kyono K.. A successful birth of healthy monozygotic dichorionic diamniotic (DD) twins of the same gender following a single vitrified-warmed blastocyst transfer. J Assist Reprod Genet 2012;29:255–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sills ES, Moomjy M, Zaninovic N, Veeck LL, McGee M, Palermo GD, Rosenwaks Z.. Human zona pellucida micromanipulation and monozygotic twinning frequency after IVF. Hum Reprod 2000;15:890–895. [DOI] [PubMed] [Google Scholar]
- Skiadas CC, Missmer SA, Benson CB, Gee RE, Racowsky C.. Risk factors associated with pregnancies containing a monochorionic pair following assisted reproductive technologies. Hum Reprod 2008;23:1366–1371. [DOI] [PubMed] [Google Scholar]
- Sobek A Jr, Zbořilová B, Procházka M, Šilhánová E, Koutná O, Klásková E, Tkadlec E, Sobek A.. High incidence of monozygotic twinning after assisted reproduction is related to genetic information, but not to assisted reproduction technology itself. Fertil Steril 2015;103:756–760. [DOI] [PubMed] [Google Scholar]
- Song J, Zhang J, Yuan X, Liu B, Tao W, Zhang C, Wu K.. Functional substitution of zona pellucida with modified sodium hyaluronate gel in human embryos. J Assist Reprod Genet 2022;39:2669–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman G. Mechanisms of twinning. II. Laterality and intercellular bonding in monozygotic twinning. J Reprod Med 2001a;46:473–479. [PubMed] [Google Scholar]
- Steinman G. Mechanisms of twinning. IV. Sex preference and lactation. J Reprod Med 2001b;46:1003–1007. [PubMed] [Google Scholar]
- Steinman G. Mechanisms of twinning. VI. Genetics and the etiology of monozygotic twinning in in vitro fertilization. J Reprod Med 2003;48:583–590. [PubMed] [Google Scholar]
- Steinman G, Valderrama E.. Mechanisms of twinning. III. Placentation, calcium reduction and modified compaction. J Reprod Med 2001;46:995–1002. [PubMed] [Google Scholar]
- Sun Y, Zeng Y, Chen H, Zhou Z, Fu J, Sang Q, Wang L, Sun X, Chen B, Xu C.. A novel homozygous variant in ZP2 causes abnormal zona pellucida formation and female infertility. J Assist Reprod Genet 2021;38:1239–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram V, Ribeiro S, Noel M.. Multi-chorionic pregnancies following single embryo transfer at the blastocyst stage: a case series and review of the literature. J Assist Reprod Genet 2018;35:2109–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland K, Leitch J, Lyall H, Woodward BJ.. Time-lapse imaging of inner cell mass splitting with monochorionic triamniotic triplets after elective single embryo transfer: a case report. Reprod Biomed Online 2019;38:491–496. [DOI] [PubMed] [Google Scholar]
- Swain JE. Fused blastocysts as a consequence of group embryo culture: observations, complications, and potential solutions. F S Rep 2021;2:133–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Széll A, Hudson RH.. Factors affecting the survival of bisected sheep embryos in vivo. Theriogenology 1991;36:379–387. [DOI] [PubMed] [Google Scholar]
- Takehara I, Takahashi T, Hara S, Matsuo K, Igarashi H, Kurachi H.. Dizygotic twin pregnancy after single embryo transfer: a case report and review of the literature. J Assist Reprod Genet 2014;31:443–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandberg A, Bjørge T, Børdahl PE, Skjaerven R.. Increasing twinning rates in Norway, 1967-2004: the influence of maternal age and assisted reproductive technology (ART). Acta Obstet Gynecol Scand 2007;86:833–839. [DOI] [PubMed] [Google Scholar]
- Tang HH, Tsai YC, Kuo CT.. Embryo splitting can increase the quantity but not the quality of blastocysts. Taiwan J Obstet Gynecol 2012;51:236–239. [DOI] [PubMed] [Google Scholar]
- Tarkowski AK, Wojewodzka M.. A method for obtaining chimaeric mouse blastocysts with two separate inner cell masses: a preliminary report. Development 1982;71:215–221. [PubMed] [Google Scholar]
- Toledo MG. Is there increased monozygotic twinning after assisted reproductive technology? Aust N Z J Obstet Gynaecol 2005;45:360–364. [DOI] [PubMed] [Google Scholar]
- Tsunoda Y, Tokunaga T, Sugie T, Katsumata M.. Production of monozygotic twins following the transfer of bisected embryos in the goats. Theriogenology 1985;24:337–343. [DOI] [PubMed] [Google Scholar]
- Ueno S, Bodri D, Uchiyama K, Okimura T, Okuno T, Kobayashi T, Kato K.. Developmental potential of zona pellucida-free oocytes obtained following mild in vitro fertilization. Fertil Steril 2014;102:1602–1607. [DOI] [PubMed] [Google Scholar]
- Vajta G, Korösi T, Du Y, Nakata K, Ieda S, Kuwayama M, Nagy ZP.. The Well-of-the-Well system: an efficient approach to improve embryo development. Reprod Biomed Online 2008;17:73–81. [DOI] [PubMed] [Google Scholar]
- Vajta G, Peura TT, Holm P, P�Ldi A, Greve T, Trounson AO, Callesen H.. New method for culture of zona-included or zona-free embryos: the Well of the Well (WOW) system. Mol Reprod Dev 2000;55:256–264. [DOI] [PubMed] [Google Scholar]
- Vajta G, Rienzi L, Bavister BD.. Zona-free embryo culture: is it a viable option to improve pregnancy rates? Reprod Biomed Online 2010;21:17–25. [DOI] [PubMed] [Google Scholar]
- van der Hoorn ML, Helmerhorst F, Claas F, Scherjon S.. Dizygotic twin pregnancy after transfer of one embryo. Fertil Steril 2011;95:805.e1–805.e3. [DOI] [PubMed] [Google Scholar]
- van Dongen J, Gordon SD, McRae AF, Odintsova VV, Mbarek H, Breeze CE, Sugden K, Lundgren S, Castillo-Fernandez JE, Hannon E. et al. ; Genetics of DNA Methylation Consortium. Identical twins carry a persistent epigenetic signature of early genome programming. Nat Commun 2021;12:5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen J, Hubers N, Boomsma DI.. New insights into the (epi)genetics of twinning. Hum Reprod 2024;39:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Langendonckt A, Wyns C, Godin PA, Toussaint-Demylle D, Donnez J.. Atypical hatching of a human blastocyst leading to monozygotic twinning: a case report. Fertil Steril 2000;74:1047–1050. [DOI] [PubMed] [Google Scholar]
- Vela G, Luna M, Barritt J, Sandler B, Copperman AB.. Monozygotic pregnancies conceived by in vitro fertilization: understanding their prognosis. Fertil Steril 2011;95:606–610. [DOI] [PubMed] [Google Scholar]
- Vitthala S, Gelbaya TA, Brison DR, Fitzgerald CT, Nardo LG.. The risk of monozygotic twins after assisted reproductive technology: a systematic review and meta-analysis. Hum Reprod Update 2009;15:45–55. [DOI] [PubMed] [Google Scholar]
- Vitucci A, Fichera A, Fratelli N, Sartori E, Prefumo F.. Twin reversed arterial perfusion sequence: current treatment options. Int J Womens Health 2020;12:435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed I. Monozygotic twin formation in mouse embryos in vitro. J Duhok Univ 2009;12:167–172. [Google Scholar]
- Wang J, Wang L, Feng G, Wang Y, Li Y, Li X, Liu C, Jiao G, Huang C, Shi J. et al. Asymmetric expression of LincGET biases cell fate in two-cell mouse embryos. Cell 2018;175:1887–1901.e18. [DOI] [PubMed] [Google Scholar]
- Waszak M, Cieślik K, Skrzypczak-Zielińska M, Szalata M, Wielgus K, Zakerska-Banaszak O, Bręborowicz G, Słomski R.. Effects of intrauterine environment on the magnitude of differences within the pairs of monozygotic and dizygotic twins. Twin Res Hum Genet 2017;20:72–83. [DOI] [PubMed] [Google Scholar]
- Watson K, Korman I, Liu Y, Zander-Fox D.. Live birth in a complete zona-free patient: a case report. J Assist Reprod Genet 2021;38:1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherbee BAT, Gantner CW, Iwamoto-Stohl LK, Daza RM, Hamazaki N, Shendure J, Zernicka-Goetz M.. A model of the post-implantation human embryo derived from pluripotent stem cells. Nature 2023;622:584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willadsen SM. The viability of early cleavage stages containing half the normal number of blastomeres in the sheep. J Reprod Fertil 1980;59:357–362. [DOI] [PubMed] [Google Scholar]
- Willadsen SM. Cloning of sheep and cow embryos. Genome 1989;31:956–962. [DOI] [PubMed] [Google Scholar]
- Willadsen SM, Godke RA.. A simple procedure for the production of identical sheep twins. Vet Rec 1984;114:240–243. [DOI] [PubMed] [Google Scholar]
- Willadsen SM, Polge C.. Attempts to produce monozygotic quadruplets in cattle by blastomere separation. Vet Rec 1981;108:211–213. [DOI] [PubMed] [Google Scholar]
- Williams TJ, Elsden RP, Seidel GE Jr. Pregnancy rates with bisected bovine embryos. Theriogenology 1984;22:521–531. [DOI] [PubMed] [Google Scholar]
- Wu D, Huang SY, Wu HM, Chen CK, Soong YK, Huang HY.. Monozygotic twinning after in vitro fertilization/intracytoplasmic sperm injection treatment is not related to advanced maternal age, intracytoplasmic sperm injection, assisted hatching, or blastocyst transfer. Taiwan J Obstet Gynecol 2014;53:324–329. [DOI] [PubMed] [Google Scholar]
- Yan Z, Liang H, Deng L, Long H, Chen H, Chai W, Suo L, Xu C, Kuang Y, Wu L. et al. Eight-shaped hatching increases the risk of inner cell mass splitting in extended mouse embryo culture. PLoS One 2015;10:e0145172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Wei Y, Duan J, Schmitz DA, Sakurai M, Wang L, Wang K, Zhao S, Hon GC, Wu J.. Blastocyst-like structures generated from human pluripotent stem cells. Nature 2021;591:620–626. [DOI] [PubMed] [Google Scholar]
- Zeng J, Sun Y, Zhang J, Wu X, Wang Y, Quan R, Song W, Guo D, Wang S, Chen J. et al. Identification of zona pellucida defects revealed a novel loss-of-function mutation in ZP2 in humans and rats. Front Endocrinol (Lausanne) 2023;14:1169378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenker J, White MD, Gasnier M, Alvarez YD, Lim HYG, Bissiere S, Biro M, Plachta N.. Expanding actin rings zipper the mouse embryo for blastocyst formation. Cell 2018;173:776–791.e17. [DOI] [PubMed] [Google Scholar]
- Zenker J, White MD, Templin RM, Parton RG, Thorn-Seshold O, Bissiere S, Plachta N.. A microtubule-organizing center directing intracellular transport in the early mouse embryo. Science 2017;357:925–928. [DOI] [PubMed] [Google Scholar]
- Zhang J, Jiang Z, Liu X, Meng A.. Eph/ephrin signaling maintains the boundary of dorsal forerunner cell cluster during morphogenesis of the zebrafish embryonic left-right organizer. Development 2016;143:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wang M, Yang Q, Li D, Li Z, Hu J, Jin L, Zhu L.. Can successful pregnancy be achieved and predicted from patients with identified ZP mutations? A literature review. Reprod Biol Endocrinol 2022;20:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Ni C, Wu L, Chen B, Xu Y, Zhang Z, Mu J, Li B, Yan Z, Fu J. et al. Novel mutations in ZP1, ZP2, and ZP3 cause female infertility due to abnormal zona pellucida formation. Hum Genet 2019;138:327–337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this review.