Abstract
The European Commission requested the EFSA Panel on Plant Health to prepare and deliver risk assessments for commodities listed in Commission Implementing Regulation (EU) 2018/2019 as ‘High risk plants, plant products and other objects’. This Scientific Opinion covers plant health risks posed by plants of Betula pendula and B. pubescens imported from the United Kingdom (UK) taking into account the available scientific information, including the technical information provided by the UK. The commodities were grouped in the risk assessment as (a) bundles of 10–20 graftwood/budwood (up to 1‐year‐old), (b) bare root plants which include bundles of 25 or 50 seedlings or transplants (1–2 years‐old), bundles of 5, 10 or 15 whips (1–2 years‐old) and single bare root plants (1–7 years‐old), (c) plants in pots which include bundles of 5 and 10 cell‐grown plants (1–2 years‐old) and rooted plants in pots (1–7 years‐old), and (d) large specimen trees up to 15‐years‐old. All pests associated with the commodities were evaluated against specific criteria for their relevance for this opinion. Two EU quarantine pests i.e. Meloidogyne fallax and Phytophthora ramorum (non‐EU isolates) and two protected zone quarantine pests i.e. Entoleuca mammata and Thaumetopoea processionea fulfilled all relevant criteria and were selected for further evaluation. For the selected pests, the risk mitigation measures described in the technical dossier from the UK were evaluated considering the possible limiting factors. For these pests an expert judgement is given on the likelihood of pest freedom taking into consideration the risk mitigation measures acting on the pest, including uncertainties associated with the assessment. In the assessment of risk, the age of the plants was considered, as larger trees are more likely to be infested mainly due to longer time grown in the field. In addition, larger canopies and root systems are more difficult to inspect, thereby making the detection of pests more challenging on large trees. The likelihood of pest freedom varies among the pests evaluated, with M. fallax being the pest most frequently expected on the imported plants. The Expert Knowledge Elicitation (EKE) indicated with 95% certainty that between 9735 and 10,000 per 10,000 large specimen trees will be free from M. fallax.
Keywords: birch, commodity risk assessment, European Union, plant health, plant pest
1. INTRODUCTION
1.1. Background and Terms of Reference as provided by European Commission
1.1.1. Background
The Plant Health Regulation (EU) 2016/2031, 1 on the protective measures against pests of plants, has been applied from December 2019. Provisions within the above Regulation are in place for the listing of ‘high risk plants, plant products and other objects’ (Article 42) on the basis of a preliminary assessment, and to be followed by a commodity risk assessment. A list of ‘high risk plants, plant products and other objects’ has been published in Regulation (EU) 2018/2019. 2 Scientific opinions are therefore needed to support the European Commission and the Member States in the work connected to Article 42 of Regulation (EU) 2016/2031, as stipulated in the terms of reference.
1.1.2. Terms of Reference
In view of the above and in accordance with Article 29 of Regulation (EC) No 178/2002, 3 the Commission asks EFSA to provide scientific opinions in the field of plant health.
In particular, EFSA is expected to prepare and deliver risk assessments for commodities listed in the relevant Implementing Act as ‘High risk plants, plant products and other objects’. Article 42, paragraphs 4 and 5, establishes that a risk assessment is needed as a follow‐up to evaluate whether the commodities will remain prohibited, removed from the list and additional measures will be applied or removed from the list without any additional measures. This task is expected to be on‐going, with a regular flow of dossiers being sent by the applicant required for the risk assessment.
Therefore, to facilitate the correct handling of the dossiers and the acquisition of the required data for the commodity risk assessment, a format for the submission of the required data for each dossier is needed.
Furthermore, a standard methodology for the performance of ‘commodity risk assessment’ based on the work already done by Member States and other international organizations needs to be set.
In view of the above and in accordance with Article 29 of Regulation (EC) No 178/2002, the Commission asks EFSA to provide scientific opinion in the field of plant health for Betula pendula and B. pubescens from the UK taking into account the available scientific information, including the technical dossier provided by the UK.
1.2. Interpretation of the Terms of Reference
The EFSA Panel on Plant Health (hereafter referred to as ‘the Panel’) was requested to conduct a commodity risk assessment of Betula pendula and B. pubescens from the UK following the Guidance on commodity risk assessment for the evaluation of high‐risk plant dossiers (EFSA PLH Panel, 2019a), taking into account the available scientific information, including the technical information provided by the UK.
The EU quarantine pests that are regulated as a group in the Commission Implementing Regulation (EU) 2019/2072 4 were considered and evaluated separately at species level.
Annex II of Implementing Regulation (EU) 2019/2072 lists certain pests as non‐European populations or isolates or species. These pests are regulated quarantine pests. Consequently, the respective European populations, or isolates, or species are non‐regulated pests.
Annex VII of the same Regulation, in certain cases (e.g. point 32) makes reference to the following countries that are excluded from the obligation to comply with specific import requirements for those non‐European populations, or isolates, or species: Albania, Andorra, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, Canary Islands, Faeroe Islands, Georgia, Iceland, Liechtenstein, Moldova, Monaco, Montenegro, North Macedonia, Norway, Russia (only the following parts: Central Federal District (Tsentralny federalny okrug), Northwestern Federal District (SeveroZapadny federalny okrug), Southern Federal District (Yuzhny federalny okrug), North Caucasian Federal District (Severo‐Kavkazsky federalny okrug) and Volga Federal District (Privolzhsky federalny okrug), San Marino, Serbia, Switzerland, Türkiye, Ukraine and the United Kingdom (except Northern Ireland 5 )).
Consequently, for those countries,
any pests identified, which are listed as non‐ European species in Annex II of Implementing Regulation (EU) 2019/2072 should be investigated as any other non‐regulated pest.
any pest found in a European country that belongs to the same denomination as the pests listed as non‐European populations or isolates in Annex II of Implementing Regulation (EU) 2019/2072, should be considered as European populations or isolates and should not be considered in the assessment of those countries.
Pests listed as ‘Regulated Non‐Quarantine Pest’ (RNQP) in Annex IV of the Commission Implementing Regulation (EU) 2019/2072, and deregulated pests (i.e. pests which were listed as quarantine pests in the Council Directive 2000/29/EC and were deregulated by Commission Implementing Regulation (EU) 2019/2072) were not considered for further evaluation. In case a pest is at the same time regulated as a RNQP and as a Protected Zone Quarantine pest, in this Opinion it should be evaluated as Quarantine pest.
In its evaluation the Panel:
Checked whether the provided information in the technical dossier (hereafter referred to as ‘the Dossier’) provided by the applicant (United Kingdom, Department for Environment Food and Rural Affairs – hereafter referred to as ‘DEFRA’) was sufficient to conduct a commodity risk assessment. When necessary, additional information was requested from the applicant.
Selected the relevant Union quarantine pests and protected zone quarantine pests (as specified in Commission Implementing Regulation (EU) 2019/2072, hereafter referred to as ‘EU quarantine pests’) and other relevant pests present in the UK and associated with the commodity.
Did not assess the effectiveness of measures for Union quarantine pests for which specific measures are in place for the import of the commodity from the UK in Commission Implementing Regulation (EU) 2019/2072 and/or in the relevant legislative texts for emergency measures and if the specific country is in the scope of those emergency measures. The assessment was restricted to whether or not the applicant country implements those measures.
Assessed the effectiveness of the measures described in the Dossier for those Union quarantine pests for which no specific measures are in place for the importation of the commodity from the UK and other relevant pests present in the UK and associated with the commodity.
Risk management decisions are not within EFSA's remit. Therefore, the Panel provided a rating based on expert judgement regarding the likelihood of pest freedom for each relevant pest given the risk mitigation measures proposed by DEFRA of the UK.
2. DATA AND METHODOLOGIES
2.1. Data provided by DEFRA of the UK
The Panel considered all the data and information (hereafter called ‘the Dossier’) provided by DEFRA of the United Kingdom (UK) in April and May 2023 including the additional information provided on 18 March 2024, after EFSA's request. The Dossier is managed by EFSA.
The structure and overview of the Dossier is shown in Table 1. The number of the relevant section is indicated in the Opinion when referring to a specific part of the Dossier.
TABLE 1.
Structure and overview of the Dossier.
| Dossier section | Overview of contents | Filename |
|---|---|---|
| 1.1 | Technical dossier for Betula pendula | Betula pendula commodity information final |
| 1.2 | Technical dossier for Betula pubescens | Betula pubescens commodity information amendment May 2023 |
| 2.0 | Pest list | Betula_Pest_List_Final |
| 3.1 | Producers sample product list for Betula pendula | Betula_pendula_producers_sample_product_list |
| 3.2 | Producers sample product list for Betula pubescens | Betula_pubescens_producers_sample_product_list |
| 4.1 | Distribution of Betula pendula plants | Betula_pendula_distribution_map |
| 4.2 | Distribution of Betula pubescens plants | Betula_pubescens_distribution_map |
| 5.1 | Additional information: answers | Betulas additional information 1 February 2024 |
| 5.2 | Additional information: pests | Defra_responses_to_EFSA_queries (1) |
| 5.3 | Additional information: answers | Betulas additional information 11 April 2024 |
The data and Supporting Information provided by DEFRA of the UK formed the basis of the commodity risk assessment. Table 2 shows the main data sources used by DEFRA of the UK to compile the Dossier (Dossier Sections 1.1, 1.2, 2.0, 3.1, 3.2, 4.1, 4.2, 5.1, 5.2 and 5.3).
TABLE 2.
Databases used in the literature searches by DEFRA of the UK.
2.2. Literature searches performed by EFSA
Literature searches in different databases were undertaken by EFSA to complete a list of pests potentially associated with Betula pendula and B. pubescens. The following searches were combined: (i) a general search to identify pests reported on B. pendula and B. pubescens in the databases, (ii) a search to identify any EU quarantine pest reported on Betula as genus and subsequently (iii) a tailored search to identify whether the above pests are present or not in the UK. The searches were run between November 2023 and January 2024 by using the databases listed in Table 3. No language, date or document type restrictions were applied in the search strategy. As for Web of Science, the literature search was performed using a specific, ad hoc established search string (see Appendix B). The string was run in ‘All Databases’ with no range limits for time or language filters. This is further explained in Section 2.3.2.
TABLE 3.
Databases used by EFSA for the compilation of the pest list associated with Betula pendula and B. pubescens.
Additional articles were considered based on references in relevant papers retrieved in the searches. The available scientific information, including previous EFSA opinions on the relevant pests and diseases (see pest data sheets in Appendix A) and the relevant literature and legislation (e.g. Regulation (EU) 2016/2031; Commission Implementing Regulations (EU) 2018/2019; (EU) 2018/2018 and (EU) 2019/2072) were taken into account.
2.3. Methodology
When developing the Opinion, the Panel followed the EFSA Guidance on commodity risk assessment for the evaluation of high‐risk plant dossiers (EFSA PLH Panel, 2019a).
In the first step, pests potentially associated with the commodity in the country of origin (EU‐quarantine pests and other pests) that may require risk mitigation measures are identified. The EU non‐quarantine pests not known to occur in the EU were selected based on evidence of their potential impact in the EU. At the end of this first step, all the relevant pests that may need risk mitigation measures were identified.
In the second step, the implemented risk mitigation measures for each relevant pest were evaluated.
In the final step, a conclusion on the pest‐freedom status of the commodity for each of the relevant pests was drawn and uncertainties identified using expert judgements.
Pest freedom was assessed by estimating the number of infested/infected units out of 10,000 exported units. Further details on the methodology used to estimate the likelihood of pest freedom are provided in Section 2.3.4.
2.3.1. Commodity data
Based on the information provided by DEFRA of the UK, the characteristics of each commodity were summarised.
2.3.2. Identification of pests potentially associated with each commodity
All plant pests reported as associated with B. pendula and B. pubescens commodities were identified based on information provided in the Dossier Sections 1.1, 1.2, 2.0, 3.1, 3.2, 4.1, 4.2, 5.1, 5.2 and 5.3 and on searches performed by the Panel. The search strategy and search syntax were adapted to each of the databases listed in Table 3, according to the options and functionalities of the different databases and CABI keyword thesaurus.
The scientific names of the host plant (i.e. B. pendula and B. pubescens) were used when searching in the EPPO Global database and CABI Crop Protection Compendium. The same strategy was applied to the other databases excluding EUROPHYT and Web of Science.
EUROPHYT was investigated by searching for the interceptions associated with B. pendula and B. pubescens imported from the whole world from 1995 to May 2020 and TRACES‐NT from May 2020 to 31 January 2024, respectively. For the pests selected for further evaluation, a search in the EUROPHYT and/or TRACES‐NT was performed for the years between 1995 and 31 January 2024 for the interceptions from the whole world, at species level.
The search strategy used for Web of Science Databases was designed combining English common names for pests and diseases, terms describing symptoms of plant diseases and the scientific and English common names of the commodity and excluding pests which were identified using searches in other databases. The established search strings are detailed in Appendix B and they were run on 21 December 2023.
The titles and abstracts of the scientific papers retrieved were screened and the pests associated with B. pendula and B. pubescens were included in the pest list. The pest list was eventually further compiled with other relevant information (e.g. EPPO code per pest, taxonomic information, categorisation, distribution) useful for the selection of the pests relevant for the purposes of this Opinion.
The compiled pest list (see Microsoft Excel® in Appendix F) includes all identified pests that use as host B. pendula and B. pubescens.
The evaluation of the compiled pest list was done in two steps: first, the relevance of the EU‐quarantine pests was evaluated (Section 4.1); second, the relevance of any other plant pest was evaluated (Section 4.2).
Pests for which limited information was available on one or more criteria used to identify them as relevant for this Opinion, e.g. on potential impact, are listed in Appendix E (List of pests that can potentially cause an effect not further assessed).
2.3.3. Listing and evaluation of risk mitigation measures
All implemented risk mitigation measures were listed and evaluated. When evaluating the likelihood of pest freedom of the commodity, the following types of potential infection/infestation sources for B. pendula and B. pubescens in export nursery were considered (see also Figure 1):
pest entry from surrounding areas,
pest entry with new plants/seeds,
pest spread within the nursery.
FIGURE 1.
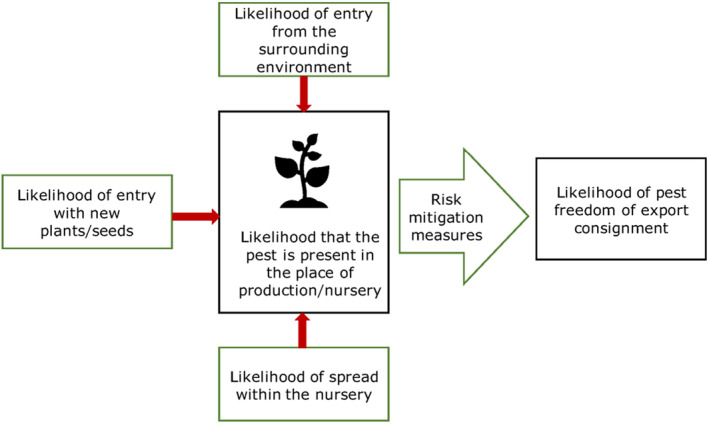
Conceptual framework to assess likelihood that plants are exported free from relevant pests (Source: EFSA PLH Panel, 2019a).
The risk mitigation measures proposed by DEFRA of the UK were evaluated with Expert Knowledge Elicitation (EKE) according to the Guidance on uncertainty analysis in scientific assessment (EFSA Scientific Committee, 2018).
Information on the biology, likelihood of entry of the pest to the export nursery, of its spread inside the nursery and the effect of measures on the specific pests were summarised in data sheets of pests selected for further evaluation (see Appendix A).
2.3.4. Expert Knowledge Elicitation
To estimate the pest freedom of the commodities an EKE was performed following EFSA guidance (Annex B.8 of EFSA Scientific Committee, 2018). The specific question for EKE was: ‘Taking into account (i) the risk mitigation measures in place in the nurseries and (ii) other relevant information, how many of 10,000 commodity units will be infested with the relevant pest when arriving in the EU?’. A unit is defined as either single plants or bundles of plants, bare rooted or potted, depending on the commodity.
For the purpose of the EKE, the commodities (see Section 3.1) were grouped as follows:
Graftwood/budwood in bundles of 10–20 (up to 1‐year‐old).
Bare root plants which include bundles of 25 or 50 seedlings or transplants (1–2 years‐old), bundles of 5, 10 or 15 whips (1–2 years‐old) and 1–7 years‐old single bare root plants.
Plants in pots which include bundles of 5 and 10 cell‐grown plants (1–2 years‐old) and single rooted plants in pots (1–7 years‐old). Single cell‐grown plants are considered covered by rooted plants in pots.
Large specimen trees 7 to 15 years‐old in pots. Specimen trees up to 7 years‐old as described in the Dossier are considered covered by the category above, rooted plants in pots.
The following reasoning is given for considering bundles of bud−/graftwood, whips and seedlings or transplants:
There is no quantitative information available regarding clustering of plants during production;
Plants are grouped in bundles after sorting;
For the pests under consideration, a cross‐contamination during transport is possible.
The following reasoning is given for grouping into bare root plants, plants in pots and large specimen trees:
Plants in pots can have leaves when exported while bare root plants are usually without leaves. Due to the absence of growing media and similar time of harvesting and export, bundles of whips and transplants and single bare‐rooted plants are considered to have a comparable risk regarding the presence of pests.
Cell‐grown plants in bundles are comparable to single plants in pots with regard to the risk of pests being present on the leaves and on the roots. The overall canopy and root volume of cell‐grown plants in bundles can be similar to that of single plants in pots. Both commodities can be exported all year round.
Large specimen trees of up to 15 years‐old can be grown in the field up to 9 years and have a much larger canopy and root volume compared to smaller plants in pots. Large specimen trees are more difficult to inspect and hence the risk of overlooking pests is greater compared to smaller plants in pots.
The uncertainties associated with the EKE were taken into account and quantified in a probability distribution fitted to the elicited percentiles, applying the semi‐formal method described in Section 3.5.2 of the EFSA‐PLH Guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018). Finally, the EKE results were reported in terms of the likelihood of pest freedom, calculated by 1 minus the likelihood to be infested. The lower 5% percentile of the uncertainty distribution reflects the opinion that pest freedom is with 95% certainty above this limit.
3. COMMODITY DATA
3.1. Description of the commodity
The commodities to be imported from the UK to the EU are graftwood/budwood, whips or transplants, bare root plants, cell‐grown plants, rooted plants in pots and large specimen trees in pots of B. pendula (common names: clump birch, common birch, European white birch, silver birch; Family: Betulaceae) and B. pubescens (common names: common birch, downy birch, swamp birch, white birch; Family: Betulaceae). There are various varieties of B. pendula and B. pubescens (Dossier Sections 1.1 and 1.2).
The commodities are as follows:
Bundles of graftwood/budwood: the age of graftwood/budwood is up to 1 year. The diameter is between 0.4 and 1.2 cm and height 40 cm. The commodity will be exported dormant, without leaves from January to March (Dossier Sections 1.1, 1.2 and 5.1).
Single plants in pots or bundles of cell‐grown plants: the age of plants is between 1 and 2 years. The diameter is between 0.4 and 1 cm and height between 20 and 60 cm. The cell‐grown plants may be exported with leaves based on the picture ‘cell‐grown plants bundled and ready for dispatch’ provided by the applicant country (Dossier Sections 1.1, 1.2 and 5.1).
Bundles of bare root whips and transplants: the age of plants is between 1 and 2 years. The diameter is between 0.4 and 1 cm and height between 80 and 120 cm. Whips are slender, unbranched trees and are either bare root or containerised (Dossier Sections 1.1 and 1.2). Transplants are plants which have been transplanted usually from seedlings less than 1‐year‐old. They can be anything from circa 20 to 150 cm tall. Transplants have stronger and more developed root systems compared to whips (Dossier Section 5.1). Bare root plants may have some leaves at the time of export, in particular when exported in early winter (Dossier Sections 1.1 and 1.2).
Bare root single plants: the age of plants is between 1 and 7 years. The diameter is between 0.4 and 4 cm and height between 80 and 200 cm. Bare root plants may have some leaves at the time of export, in particular when exported in early winter (Dossier Sections 1.1 and 1.2).
Single plants in pots: the age of plants is from 1 to 7 years. The diameter range between 0.4 and 4 cm and the height between 80 and 250 cm. The plants in pots may be exported with leaves, depending on the timing of the export (Dossier Sections 1.1, 1.2 and 5.1).
Single large specimen trees in pots: the age of plants is up to 15 years. The diameter is up to 20 cm and height up to 600 cm. The plants in pots may be exported with leaves, depending on the timing of the export (Dossier Sections 1.1, 1.2 and 5.1).
The growing media is virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) (Dossier Sections 1.1 and 1.2) complying with the requirements for growing media as specified in the Annex VII of the Commission Implementing Regulation 2019/2072.
According to ISPM 36 (FAO, 2019), the commodities can be classified as ‘budwood/graftwood’, ‘bare root plants’ and ‘rooted plants in pots’.
According to the Dossier Section 1.1, the trade volume of B. pendula is up to 500 graftwood, 500,000 bare root plants and 100,000 rooted plants in pots (including cell‐grown plants) per year. According to the Dossier Section 1.2, the trade volume of B. pubescens is up to 2000 graftwood, 450,000 bare root plants and 110,000 rooted plants in pots (including cell‐grown plants) per year (see Table 4). The trade of these plants will mainly be to Northern Ireland and the Republic of Ireland. No information is provided on the trade volume of large specimen trees.
TABLE 4.
Trade volumes of Betula pendula and B. pubescens commodities.
| Type of plant | Number of items | Seasonal timing |
|---|---|---|
| Betula pendula | ||
| Graftwood | 500 | January to March |
| Bare‐rooted plants | 500,000 | November to April |
| Rooted plants in pots (including cell‐grown plants) | 100,000 | Mainly September to May |
| Betula pubescens | ||
| Bare‐rooted plants | 450,000 | November to April |
| Rooted plants in pots (including cell‐grown plants) | 110,000 | Mainly September to May |
According to the Dossier Sections 1.1 and 1.2, the intended use of the commodities is as follows. Plants are supplied directly to professional operators and traders. Uses may include propagation, growing‐on, onward trading or onward sales to final customers but will generally fall into the following categories:
Tree production and further growing‐on by professional operators;
Direct sales to final users as ornamentals;
Landscapers, mainly for woodland and ornamental/landscape planting.
3.2. Description of the production areas
There are six known nurseries in the UK that are producing B. pendula plants for the export to the EU (Dossier Section 1.1). The locations of these nurseries are shown in Figure 2.
FIGURE 2.
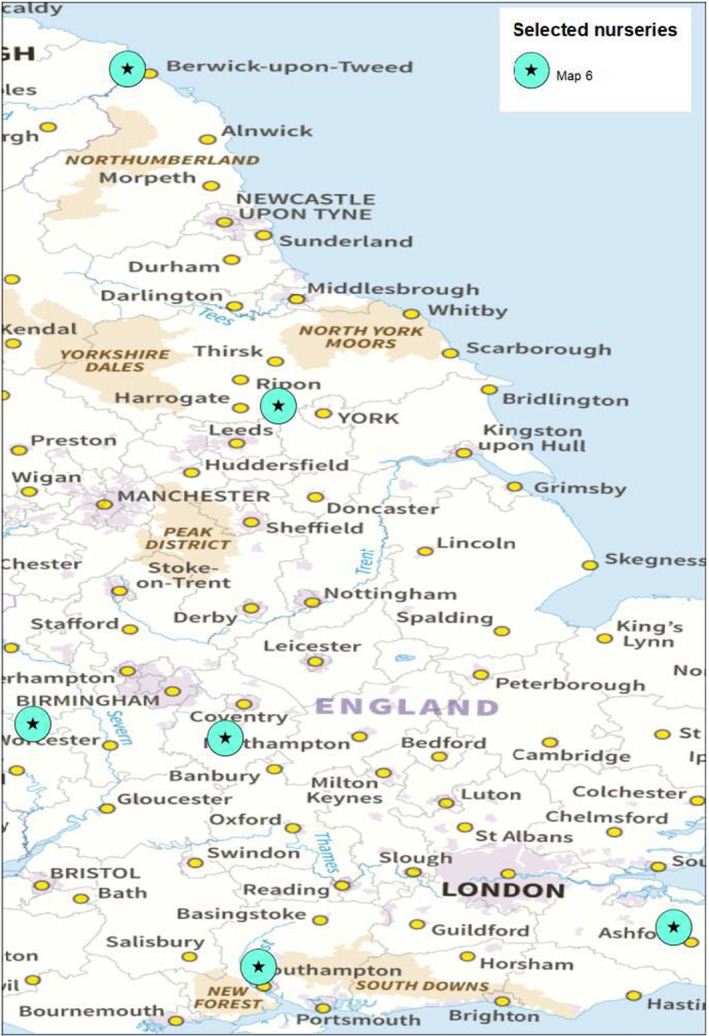
Location of the nurseries in the UK producing B. pendula plants for export to the EU (Source: Dossier Section 1.1).
Out of the above‐mentioned nurseries producing B. pendula, for export, five produce also B. pubescens (Dossier Section 1.2). The locations of these nurseries are shown in Figure 3.
FIGURE 3.
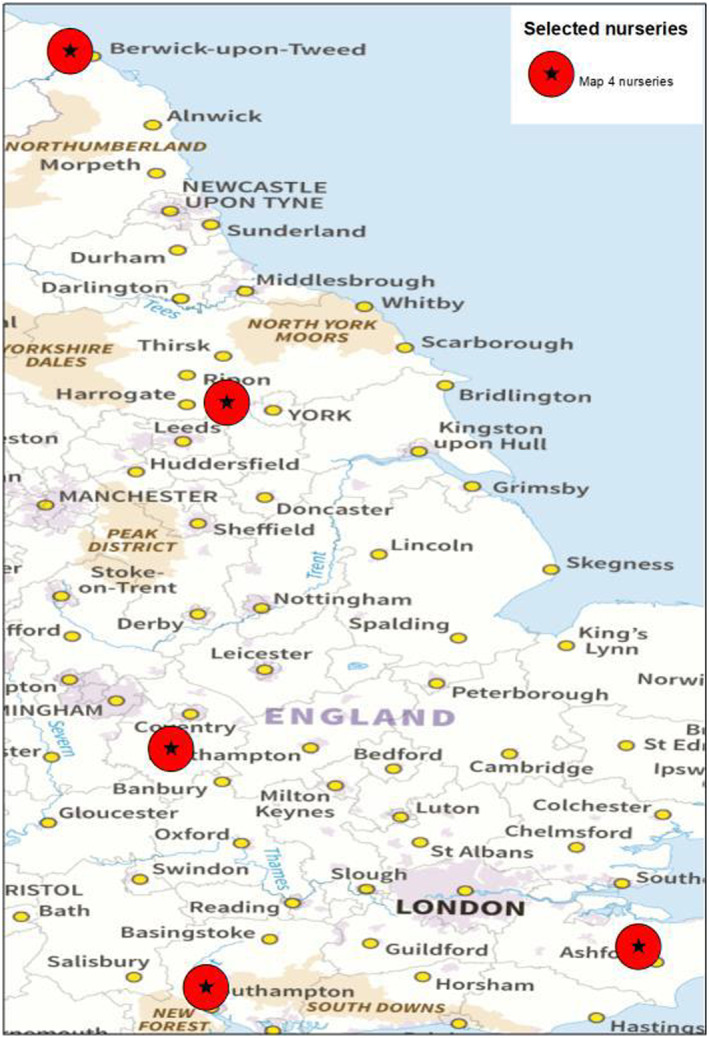
Location of the nurseries in the UK producing B. pubescens plants for export to the EU (Source: Dossier Section 1.2).
The coordinates of the Betula nurseries are provided in Table 5.
TABLE 5.
Coordinates of Betula nurseries according to the Dossier Section 5.1.
| Nursery | Longitude | Latitude |
|---|---|---|
| 1 | −1.60542 | 52.22817 |
| 2 | −1.42654 | 51.01123 |
| 3 | −2.12298 | 55.78782 |
| 4 | 0.782458 | 51.22164 |
| 5 | −2.62551 | 52.30226 |
| 6 | −1.32179 | 53.99612 |
Betula species are grown in Great Britain in line with the Plant Health (Amendment etc.) (EU Exit) Regulations 2020 6 and the Plant Health (Phytosanitary Conditions) (Amendment) (EU Exit) Regulations 2020. 7 These regulations are broadly similar to the EU phytosanitary regulations. All plants within the UK nurseries are grown under the same phytosanitary measures, meeting the requirements of the UK Plant Passporting regime (Dossier Sections 1.1 and 1.2).
The size of the nurseries is between 8 and 150 ha for container stock (plants in pots) and up to 325 ha for field grown stock (Dossier Sections 1.1 and 1.2).
The nurseries also grow other plant species as shown in the Appendix C. The minimum and maximum proportion of Betula compared to the other plant species grown in the nurseries is between 1% and 15% for B. pendula and between 1% and 3% for B. pubescens. There are nurseries which also produce plants for the local market, and there is no distancing between production areas for the export and the local market (Dossier Sections 1.1 and 1.2).
The nurseries are kept clear of non‐cultivated herbaceous plants. In access areas, non‐cultivated herbaceous plants are kept to a minimum and only exist at nursery boundaries. Non‐cultivated herbaceous plants grow on less than 1% of the nursery area. The predominant species is rye grass (Lolium spp.). Other identified species include dandelions (Taraxacum officinale), hairy bittercress (Cardamine hirsuta), common daisy (Bellis perennis), creeping cinquefoil (Potentilla reptans) and bluebells (Hyacinthoides non‐scripta). These are all extremely low in number (Dossier Sections 1.1 and 1.2).
There are hedges surrounding the export nurseries made up of a range of species including hazel (Corylus avellana), yew (Taxus baccata), holly (Ilex spp.), ivy (Hedera spp.), alder (Alnus glutinosa), cherry laurel (Prunus laurocerasus), hawthorn (Crataegus spp.), blackthorn (Prunus spinosa) and leylandii (Cupressus × leylandii) (Dossier Sections 1.1 and 1.2).
The minimum distance in a straight line, between the growing area in the nurseries and the closest B. pendula plants in the local surroundings is 200 metres and the closest B. pubescens plants in the local surroundings is 500 metres (Dossier Sections 1.1 and 1.2).
Nurseries are predominately situated in rural areas. The surrounding land tend to be arable farmland with some pasture for animals and small areas of woodland. Hedges are often used to define field boundaries and grown along roadsides (Dossier Sections 1.1 and 1.2).
Arable crops present around the nurseries are rotated in line with good farming practices and could include oilseed rape (Brassica napus), wheat (Triticum spp.), barley (Hordeum vulgare), turnips (Brassica rapa subsp. rapa), potatoes (Solanum tuberosum) and maize (Zea mays) (Dossier Sections 1.1 and 1.2).
Pastures present around the nurseries are predominantly ryegrass (Lolium spp.) (Dossier Sections 1.1 and 1.2).
Woodland is present around the nurseries. Woodlands tend to be a standard UK mixed woodland, with a range of the UK native trees such as oak (Quercus robur), pine (Pinus spp.), poplar (Populus spp.), ash (Fraxinus spp.), sycamore (Acer pseudoplatanus), holly (Ilex spp.), Norway maple (Acer platanoides) and field maple (Acer campestre). The nearest woodland to one of the nurseries borders the boundary fence (Dossier Sections 1.1 and 1.2).
It is not possible to identify the plant species growing within the gardens of private dwellings around the nurseries (Dossier Sections 1.1 and 1.2). The following plant species may be grown in some of the nurseries: Betula papyrifera, Betula lenta, Chamaecyparis lawsoniana, Larix kaempferi, Larix spp., Malus domestica, Fagus sylvatica, Fagus spp., Picea abies, Populus spp., Prunus persica, Prunus spp., Pyrus communis, Quercus petraea, Quercus robur, Quercus spp., Rhododendron spp., Rubus idaeus, Syringa vulgaris, Taxus baccata, Viburnum spp. and Vitis vinifera (Dossier Section 5.1).
The following plant species may be grown within a 2 km zone surrounding the nurseries: Allium porrum, Beta vulgaris, Betula alleghaniensis, Betula papyrifera, Betula lenta, Camellia spp., Chamaecyparis lawsoniana, Daucus carota, Hordeum vulgare, Lactuca sativa, Larix kaempferi, Larix spp., Lolium multiflorum, Malus domestica, Medicago sativa, Fagus sylvatica, Fagus spp., Pelargonium × hortorum, Picea abies, Pieris spp., Populus tremuloides, Populus spp., Prunus persica, Prunus spp., Pyrus communis, Quercus petraea, Quercus pubescens, Quercus robur, Quercus spp., Rhododendron spp., Rubus idaeus, Solanum spp., Syringa vulgaris, Taxus baccata, Trifolium repens, Viburnum spp., Vitis vinifera and Zea mays (Dossier Section 5.1).
Based on the global Köppen–Geiger climate zone classification (Kottek et al., 2006), the climate of the production areas of B. pendula and B. pubescens in the UK is classified as Cfb, i.e. main climate (C): warm temperate; precipitation (f): fully humid; temperature (b): warm summer.
3.3. Production and handling processes
3.3.1. Source of planting material
The starting material of the commodities is a mix of seeds and seedlings depending on the nursery (Dossier Sections 1.1 and 1.2).
Seeds purchased in the UK are certified under the Forest Reproductive Material (Great Britain) Regulations 2002. Seedlings sourced in the UK are certified with the UK Plant Passports. A small percentage of seedlings are obtained from EU countries (the Netherlands) and they are certified with phytosanitary certificates (Dossier Sections 1.1 and 1.2). The plant material could be sourced from a number of different suppliers, but currently from Dodewaard and Boskoop in the Netherlands (Dossier Section 5.1).
Most nurseries do not produce plants by grafting. Only one of the nurseries expected to export to the EU that produces plants from grafting holds mother plants of Betula pendula on site. The same nursery holds mother plants of other Betula species (B. alba, B. albosinensis, B. utilis, B. costata, B. ermanii, B. nigra, B. sinensis) (Dossier Sections 1.1 and 1.2).
When grafting is used, the two most common methods are ‘side‐spliced’ and ‘whip and tongue’ grafting, both of which are usually undertaken in late winter or early spring (November to February) (Dossier Sections 1.1 and 1.2).
3.3.2. Production cycle
Plants are either grown in containers (cells, pots, tubes, etc.) or in the field. Cell‐grown plants can be grown in greenhouses; however, most plants will be field grown or field grown in containers (Dossier Sections 1.1 and 1.2). The minimum distance between greenhouses and production fields of Betula is 10 m (Dossier Section 5.1).
As the plants are intended for outdoor cultivation it is normally only the early growth stages that are maintained under protection, such as young plants where there is an increased vulnerability due to climatic conditions including frost. The commodity to be exported should therefore be regarded as outdoor grown. Growth under protection is primarily to protect against external climatic conditions rather than protection from pests. The early stages of plants grown under protection are maintained in plastic polytunnels, or in glasshouses which typically consist of a metal or wood frame construction and glass panels (Dossier Sections 1.1, 1.2 and 5.1).
Rooted plants in pots may be either grown in EU‐compliant growing media in pots for their whole life or initially grown in the field before being lifted, root‐washed to remove the soil and then potted in EU‐compliant growing media. Trees will be lifted from the field, root‐washed to remove the soil and transplanted into pots at least one growing season before export (Dossier Section 5.1).
Specimen trees may either be grown in pots in EU‐compliant media their whole life or be initially grown in the field, lifted at no more than 9‐years‐old, root‐washed and subsequently grown from that point on in pots in EU‐compliant growing media. Trees will be lifted from the field at least one growing season before export (Dossier Sections 5.1 and 5.3).
Pruning is done on the different kind of commodities and its frequency depends on growth, age of plant, nursery and customer preference. The whips are not pruned (Dossier Section 5.1).
According to the Dossier Sections 1.1 and 1.2, bare root plants are harvested in winter to be able to lift plants from the field and because this is the best time to move dormant plants. Rooted plants in pots can be moved at any point in the year to fulfil customer demand.
The growing media is virgin peat or peat‐free compost. This compost is heat‐treated by commercial suppliers during production to eliminate pests and diseases. It is supplied in sealed bulk bags or shrink‐wrapped bales and stored off the ground on pallets, these are free from contamination. Where delivered in bulk, compost is kept in a dedicated bunker, either indoors or covered by tarpaulin outdoors, and with no risk of contamination with soil or other material (Dossier Sections 1.1 and 1.2).
The irrigation is done when needed and could be overhead, sub irrigation or drip irrigation. Water used for irrigation can be drawn from several sources, the mains supply, bore holes or from rainwater collection or watercourses (Dossier Sections 1.1 and 1.2). Additional information on water used for irrigation is provided in Appendix D. Regardless of the source of the water used to irrigate, none of the nurseries are known to have experienced the introduction of a pest/disease because of contamination of the water supply (Dossier Sections 1.1 and 1.2).
Growers are required to assess water sources, irrigation and drainage systems used in plant production for the potential to harbour and transmit plant pests. Water is routinely sampled and sent for analysis (Dossier Sections 1.1 and 1.2).
Growers must have an appropriate programme of weed management in place on the nursery (Dossier Sections 1.1 and 1.2).
General hygiene measures are undertaken as part of routine nursery production, including disinfection of tools and equipment between batches/lots and different plant species. The tools are dipped in a disinfectant solution and wiped with a clean cloth between trees to reduce the risk of virus and bacterial transfer between subjects. There are various disinfectants available, with Virkon S (active substance: potassium peroxymonosulfate and sodium chloride) being a common example (Dossier Sections 1.1 and 1.2).
Growers keep records to allow traceability for all plant material handled. These records must allow a consignment or consignment in transit to be traced back to the original source, as well as forward to identify all trade customers to which those plants have been supplied (Dossier Sections 1.1 and 1.2).
3.3.3. Pest monitoring during production
All producers are registered as professional operators with the UK Competent Authority via the Animal and Plant Health Agency (APHA) for England and Wales, or with Science and Advice for Scottish Agriculture (SASA) for Scotland, and are authorised to issue UK plant passports, verifying they meet the required national sanitary standards. The Competent Authority inspects crops at least once a year to check they meet the standards set out in the guides. Assessments are normally made based on visual examinations, but samples may be taken for laboratory analysis to get a definitive diagnosis (Dossier Sections 1.1 and 1.2).
The sanitary status of production areas is controlled by the producers as part of these schemes, as well as via official inspections by APHA Plant Health and Seeds Inspectors (PHSI; England and Wales) or with SASA (Scotland) (Dossier Sections 1.1 and 1.2).
In the Dossier it is reported that in the last 3 years there has been a substantial level of inspection of registered B. pendula and B. pubescens producers, both in support of the Plant Passporting scheme (checks are consistent with EU legislation, with a minimum of one a year for authorised operators) and as part of the Quarantine Surveillance programme (Great Britain uses the same framework for its surveillance programme as the EU) (Dossier Sections 1.1 and 1.2).
Plant material is regularly monitored for plant health issues. Pest monitoring is carried out by trained nursery staff via crop walking and records kept of this monitoring. Qualified agronomists also undertake crop walks to verify the producer's assessments. Curative or preventative actions are implemented together with an assessment of phytosanitary risk. Unless a pest can be immediately and definitively identified as non‐quarantine, growers are required to treat it as a suspect quarantine pest and notify the Competent Authority (Dossier Sections 1.1 and 1.2).
The crops are inspected visually on a regular basis by competent nursery staff as part of the growing process. All plants are also carefully inspected by nurseries on arrival and dispatch for any plant health issues (Dossier Sections 1.1 and 1.2).
The nurseries follow the Plant Health Management Standard issued by the Plant Healthy Certification Scheme which DEFRA, the Royal Horticultural Society and others contribute to via The Plant Health Alliance Steering Group (Dossier Sections 1.1 and 1.2).
The UK surveillance is based on visual inspection with samples taken from symptomatic material, and where appropriate, samples are also taken from asymptomatic material (e.g. plants, tubers, soil, watercourses). For sites with the likelihood of multiple pest and host combinations (e.g. ornamental and retail sites) standard methods are used for site selection and visit frequency, whereby clients are assessed taking into account business activity, size of business and source material, so for example a large propagator using third country material receives 10 visits per year whilst a small retailer selling locally sourced material is visited once every second year. Where pest specific guidelines are absent, inspectors select sufficient plants to give a 95% probability of detecting symptoms randomly distributed on 1.5% of plants in a batch/consignment. For inspections of single hosts, possibly with multiple pests, survey site selection is often directed to specific locations identified by survey planners, for example 0.5% of ware production land is annually sampled for potato cyst nematode with farms randomly selected and sampled at a rate of 50 cores per hectare (Dossier Sections 1.1 and 1.2).
During production, in addition to the general health monitoring of the plants by the nurseries, official growing season inspections are undertaken by the UK Plant Health Service at an appropriate time, taking into consideration factors such as the likelihood of pest presence and growth stage of the crop. Where appropriate this could include sampling and laboratory analysis. Official sampling and analysis could also be undertaken nearer to the point of export depending on the type of analysis and the import requirements of the country being exported to. Samples are generally taken on a representative sample of plants, in some cases however where the consignment size is quite small all plants are sampled. Magnification equipment is provided to all inspectors as part of their standard equipment and is used during inspections when appropriate (Dossier Sections 1.1 and 1.2).
All residues or waste materials are reported to be assessed for the potential to host, harbour and transmit pests (Dossier Sections 1.1 and 1.2).
Incoming plant material and other goods such as packaging material and growing media that have the potential to be infected or harbour pests, are checked on arrival. Growers have procedures in place to quarantine any suspect plant material and to report findings to the authorities (Dossier Sections 1.1 and 1.2).
3.3.4. Pest management during production
Crop protection is achieved using a combination of measures including approved plant protection products, biological control or physical measures. Plant protection products are only used when necessary and records of all plant protection treatments are kept (Dossier Sections 1.1 and 1.2).
Pest and disease pressure varies from season to season. Product application takes place only when required and depends on situation (disease pressure, growth stage etc. and environmental factors) at that time. Subject to this variation in pest pressure, in some seasons few, if any, pesticides are applied; in others it is sometimes necessary to apply preventative and/or control applications of pesticides. In many circumstances also, biological control is used to control outbreaks, rather than using chemical treatments (Dossier Sections 1.1 and 1.2).
Examples of typical treatments used against rust fungi, spider mites, aphids, caterpillars and weeds are listed in the Dossier Sections 1.1, 1.2, 5.1 and 5.2. These would be applied at the manufacturers recommended rate and intervals (Dossier Sections 1.1 and 1.2).
There are no specific measures/treatments against soil pests. However, containerised plants are grown in trays on top of protective plastic membranes to prevent contact with soil. Membranes are regularly refreshed when needed. Alternatively, plants may be grown on raised galvanised steel benches stood on gravel as a barrier between the soil and bench feet and/or concreted surfaces (Dossier Sections 1.1 and 1.2).
Post‐harvest and through the autumn and winter, nursery management is centred on pest and disease prevention and maintaining good levels of nursery hygiene. Leaves, pruning residues and weeds are all removed from the nursery to reduce the number of over wintering sites for pests and diseases (Dossier Sections 1.1 and 1.2).
3.3.5. Inspections before export
The UK NPPO carries out inspections and testing where required by the country of destination's plant health legislation, to ensure all requirements are fulfilled and a valid phytosanitary certificate with the correct additional declarations is issued (Dossier Sections 1.1 and 1.2).
Separate to any official inspection, plant material is checked by growers for plant health issues prior to dispatch (Dossier Sections 1.1 and 1.2).
A final pre‐export inspection is undertaken as part of the process of issuing a phytosanitary certificate. These inspections are generally undertaken as near to the time of export as possible, usually within 1–2 days and not more than 2 weeks before export. Phytosanitary certificates are only issued if the commodity meets the required plant health standards after inspection and/or testing according to appropriate official procedures (Dossier Sections 1.1 and 1.2).
The protocol for plants infested by pests during inspections before export is to treat the plants, if they are on site for a sufficient period of time or to destroy any plants infested by pests otherwise. All other host plants in the nursery would be treated. The phytosanitary certificate for export will not be issued until the UK Plant Health inspectors confirm that the plants are free from pests (Dossier Sections 1.1 and 1.2).
3.3.6. Export procedure
Bare‐rooted plants are harvested from autumn to early spring (October to April) to be able to lift plants from the field and because this is the best time to move dormant plants. Bare root plants are lifted and washed free from soil with a low‐pressure washer in the outdoors nursery area away from packing/cold store area. In some cases, the plants may be kept in a cold store stored for up to 5 months after harvesting prior to export (Dossier Sections 1.1 and 1.2).
Rooted plants in pots can be moved at any point in the year to fulfil customer demand. These will likely be destined for garden centre trade rather than nurseries (Dossier Sections 1.1 and 1.2).
Graftwood/budwood is wrapped in plastic and packed in cardboard boxes or Dutch crates on ISPM certified wooden pallets or metal pallets, dependant on quantity. Graftwood/budwood may be exported in bundles of 10–20 items (Dossier Sections 1.1 and 1.2).
Cell‐grown plants may be traded as individual plants or as bundles. Typically, bundles will include 5–10 plants depending on the size of plant (Dossier Section 5.1).
Prior to export bare root plants can be placed in bundles, depending on the size of the plants (25 or 50 for transplants; 5, 10 or 15 for whips; or single bare root trees). They are then wrapped in polythene and packed and distributed on ISPM 15 certified wooden pallets or metal pallets. Alternatively, they may be placed in pallets which are then wrapped in polythene. Small volume orders may be packed in waxed cardboard cartons or polythene bags and dispatched via courier (Dossier Sections 1.1 and 1.2).
Rooted plants in pots are transported on Danish trolleys for smaller containers, or ISPM 15 certified pallets, or individually in pots for larger containers (Dossier Sections 1.1 and 1.2).
The preparation of the commodities for export is carried out inside the nurseries in a closed environment, e.g. packing shed, except for the specimen trees, which are prepared outside in an open field due to their dimensions (Dossier Sections 1.1 and 1.2).
Plants are transported by lorry (size dependant on load quantity). Sensitive plants are occasionally transported by temperature‐controlled lorry if weather conditions during transit are likely to be very cold (Dossier Sections 1.1 and 1.2).
4. IDENTIFICATION OF PESTS POTENTIALLY ASSOCIATED WITH THE COMMODITY
The search for potential pests associated with the commodity rendered 1515 species (see Microsoft Excel® file in Appendix F).
4.1. Selection of relevant EU‐quarantine pests associated with the commodity
The EU listing of union quarantine pests and protected zone quarantine pests (Commission Implementing Regulation (EU) 2019/2072) is based on assessments concluding that the pests can enter, establish, spread and have potential impact in the EU.
43‐quarantine pests that are reported to use the commodity as a host plant were evaluated (Table 6) for their relevance of being included in this Opinion.
TABLE 6.
Overview of the evaluation of the 43 EU‐quarantine pest species for which information was found in the Dossier, databases and literature searches that use Betula as a host plant for their relevance for this opinion.
| No. | Pest name according to EU legislation a | EPPO code | Group | Pest present in the UK | Betula confirmed as a host (reference) | Pest can be associated with the commodity | Pest relevant for the opinion |
|---|---|---|---|---|---|---|---|
| 1 | Acleris senescens | ACLRSE | Insects | No | Betula spp. (EFSA PLH Panel, 2019b) | Not assessed | No |
| 2 | Agrilus anxius | AGRLAX | Insects | No | Betula pendula (Santamour, 1999) | Not assessed | No |
| 3 | Anoplophora chinensis | ANOLCN | Insects | No | Betula pendula (Sjöman et al., 2014) | Not assessed | No |
| 4 | Anoplophora glabripennis | ANOLGL | Insects | No | Betula pendula (Sjöman et al., 2014) | Not assessed | No |
| 5 | Choristoneura conflictana | ARCHCO | Insects | No | Betula spp. (Ciesla & Kruse, 2009) | Not assessed | No |
| 6 | Choristoneura rosaceana | CHONRO | Insects | No | Betula (Ferguson, 1975) | Not assessed | No |
| 7 | Diabrotica virgifera zeae | DIABVZ | Insects | No | Betula (Clark et al., 2004) | Not assessed | No |
| 8 | Entoleuca mammata | HYPOMA | Fungi | Yes | Betula pubescens (Granmo et al., 1999), B. alleghaniensis, B. papyrifera (Ginns, 1986) | Yes | Yes |
| 9 | Euwallacea fornicatus sensu lato | XYLBFO | Insects | No | Betula pendula (Eskalen et al., 2013) | Not assessed | No |
| 10 | Lopholeucaspis japonica | LOPLJA | Insects | No | Betula papyrifera, B. utilis (Shrewsbury et al., 2013) | Not assessed | No |
| 11 | Lycorma delicatula | LYCMDE | Insects | No | Betula pendula (Barringer & Ciafré, 2020) | Not assessed | No |
| 12 | Meloidogyne chitwoodi | MELGCH | Nematodes | No | Betula pendula (den Nijs et al., 2004) | Not assessed | No |
| 13 | Meloidogyne fallax | MELGFA | Nematodes | Yes | Betula pendula (den Nijs et al., 2004) | Yes | Yes |
| 14 | Oemona hirta | OEMOHI | Insects | No | Betula sp. (Lu & Wang, 2005) | Not assessed | No |
| 15 | Phymatotrichopsis omnivora | PHMPOM | Fungi | No | Betula nigra (Anonymous, 1960) | Not assessed | No |
| 16 |
Phytophthora ramorum (non‐EU isolates) |
PHYTRA | Oomycetes | Yes | Betula pendula (Webber et al., 2010) | Yes | Yes |
| 17 | Popillia japonica | POPIJA | Insects | No | Betula populifolia (Fleming, 1972) | Not assessed | No |
| 18 | Saperda candida | SAPECN | Insects | No | Betula sp. (Vlasak & Vlasakova, 2002) | Not assessed | No |
| 19 | Thaumetopoea processionea | THAUPR | Insects | Yes | Betula (Stigter et al., 1997) | Yes | Yes |
| 20 | Trirachys sartus | AELSSA | Insects | No | Betula sp. (Hayat, 2022) | Not assessed | No |
| 21 | Xiphinema americanum sensu stricto | XIPHAA | Nematodes | No | Betula alba (Siddiqui et al., 1973) | Not assessed | No |
| 22 |
Xiphinema rivesi (non‐EU populations) |
XIPHRI | Nematodes | No | Betula nigra (USDA, 2024) | Not assessed | No |
| Scolytinae spp. (non‐European) | |||||||
| 23 |
Alniphagus aspericollis as Scolytinae spp. (non‐European) |
ALNIAS | Insects | No | Betula occidentalis (Takaro, 2013) | Not assessed | No |
| 24 |
Ambrosiodmus obliquus as Scolytinae spp. (non‐European) |
AMBDOB | Insects | No | Betula spp. (Wood & Bright, 1992) | Not assessed | No |
| 25 | Ambrosiodmus tachygraphus as Scolytinae spp. (non‐European) | AMBDTA | Insects | No | Betula spp. (Wood & Bright, 1992) | Not assessed | No |
| 26 | Ambrosiophilus atratus as Scolytinae spp. (non‐European) | XYLBAT | Insects | No | Betula schmidtii (Atkinson, 2024) | Not assessed | No |
| 27 | Anisandrus maiche as Scolytinae spp. (non‐European) | ANIDMA | Insects | No | Betula spp. (Wood & Bright, 1992) | Not assessed | No |
| 28 | Anisandrus obesus as Scolytinae spp. (non‐European) | ANIDOB | Insects | No | Betula spp. (Wood & Bright, 1992) | Not assessed | No |
| 29 | Anisandrus sayi as Scolytinae spp. (non‐European) | ANIDSA | Insects | No | Betula spp. (Wood & Bright, 1992) | Not assessed | No |
| 30 | Cyclorhipidion pelliculosum as Scolytinae spp. (non‐European) | XYLBPL | Insects | No | Betula schmidtii (Atkinson, 2024) | Not assessed | No |
| 31 | Dryocoetes betulae as Scolytinae spp. (non‐European) | DRYOBE | Insects | No | Betula lenta, B. lutea, B. papyrifera (Wood & Bright, 1992) | Not assessed | No |
| 32 | Euwallacea validus as Scolytinae spp. (non‐European) | XYLBVA | Insects | No | Betula platyphylla var. japonica (Peng et al., 2022) | Not assessed | No |
| 33 | Heteroborips seriatus as Scolytinae spp. (non‐European) | XYLBSE | Insects | No | Betula spp. (Wood & Bright, 1992) | Not assessed | No |
| 34 | Hylocurus rudis as Scolytinae spp. (non‐European) | – | Insects | No | Betula nigra (Atkinson, 2024) | Not assessed | No |
| 35 | Hypothenemus crudiae as Scolytinae spp. (non‐European) | HYOTHI | Insects | No | Betula spp. (Wood & Bright, 1992) | Not assessed | No |
| 36 | Monarthrum mali as Scolytinae spp. (non‐European) | MNTHMA | Insects | No | Betula lutea (Wood & Bright, 1992) | Not assessed | No |
| 37 | Pseudopityophthorus asperulus as Scolytinae spp. (non‐European) | – | Insects | No | Betula populifolia (Wood & Bright, 1992) | Not assessed | No |
| 38 | Pseudopityophthorus minutissimus as Scolytinae spp. (non‐European) | PSDPMI | Insects | No | Betula spp. (Wood & Bright, 1992) | Not assessed | No |
| 39 | Scolytus dahuricus as Scolytinae spp. (non‐European) | – | Insects | No | Betula spp. (Wood & Bright, 1992) | Not assessed | No |
| 40 | Taphrorychus betulae as Scolytinae spp. (non‐European) | – | Insects | No | Betula spp. (Wood & Bright, 1992) | Not assessed | No |
| 41 | Trypodendron betulae as Scolytinae spp. (non‐European) | TRYDBE | Insects | No | Betula lenta, B. papyrifera (Wood & Bright, 1992) | Not assessed | No |
| 42 | Xyleborus ferrugineus as Scolytinae spp. (non‐European) | XYLBFE | Insects | No | Betula lutea (Wood & Bright, 1992) | Not assessed | No |
| 43 | Xyloterinus politus as Scolytinae spp. (non‐European) | XYORPO | Insects | No | Betula spp. (Wood & Bright, 1992) | Not assessed | No |
Commission Implementing Regulation (EU) 2019/2072.
The relevance of an EU‐quarantine pest for this opinion was based on evidence that:
the pest is present in the UK;
any Betula species is a host of the pest;
one or more life stages of the pest can be associated with the specified commodities.
Pests that fulfilled all criteria were selected for further evaluation. If one of the three criteria was not fulfilled the other criteria were not assessed.
Table 6 presents an overview of the evaluation of the 43 EU‐quarantine pest species that are reported as associated with the commodity.
Of these 43 EU‐quarantine pest species evaluated, 4 (Entoleuca mammata, Meloidogyne fallax, Phytophthora ramorum (non‐EU isolates) and Thaumetopoea processionea) are present in the UK and can be associated with the commodity and hence were selected for further evaluation.
4.2. Selection of other relevant pests (non‐regulated in the EU) associated with the commodity
The information provided by the UK, integrated with the search performed by EFSA, was evaluated in order to assess whether there are other potentially relevant pests potentially associated with the commodity species present in the country of export. For these potential pests that are non‐regulated in the EU, pest risk assessment information on the probability of entry, establishment, spread and impact is usually lacking. Therefore, these pests were also evaluated to determine their relevance for this Opinion based on evidence that:
the pest is present in the UK;
the pest is (i) absent or (ii) has a limited distribution in the EU;
commodity is a host of the pest;
one or more life stages of the pest can be associated with the specified commodity;
the pest may have an impact in the EU.
For non‐regulated species with a limited distribution (i.e. present in one or a few EU MSs) and fulfilling the other criteria (i.e. c, d and e), either one of the following conditions should be additionally fulfilled for the pest to be further evaluated:
official phytosanitary measures have been adopted in at least one EU MS;
any other reason justified by the working group (e.g. recent evidence of presence).
Pests that fulfilled the above listed criteria were selected for further evaluation. If one of the above criteria was not fulfilled the other criteria were not assessed. Based on the information collected, 1472 non‐regulated potential pests known to be associated with species community were evaluated for their relevance to this Opinion. Pests were excluded from further evaluation when at least one of the conditions listed above (1–5) was not met. Details can be found in the Appendix F (Microsoft Excel® file). None of the pests not regulated in the EU was selected for further evaluation because none of them met all selection criteria.
4.3. Overview of interceptions
Data on the interception of harmful organisms on plants of Betula can provide information on some of the organisms that can be present on Betula despite the current measures taken. According to EUROPHYT (2024) (accessed on 9 February 2024) and TRACES‐NT (2024) (accessed on 9 February 2024), there were no interceptions of plants for planting of Betula from the UK destined to the EU Member States due to the presence of harmful organisms between the years 1995 and 31 January 2024. It should be noted that the UK was previously part of the EU and at that time Betula was not subjected to plant passport, and that since Brexit the movement of Betula to the EU has been banned according to the current plant health legislation.
4.4. List of potential pests not further assessed
The Panel highlighted one potentially relevant pest, i.e. Acremonium apii (see Appendix E) for which, however, the impact and the association with commodities are uncertain.
4.5. Summary of pests selected for further evaluation
The four pests satisfying all the relevant criteria listed above in the Sections 4.1 and 4.2 are included in Table 7. The effectiveness of the risk mitigation measures applied to the commodity was evaluated for these selected pests.
TABLE 7.
List of relevant pests selected for further evaluation.
| Number | Current scientific name | EPPO code | Name used in the EU legislation | Taxonomic information | Group | Regulatory status |
|---|---|---|---|---|---|---|
| 1 | Entoleuca mammata | HYPOMA | Entoleuca mammata (Wahlenb.) Rogers and Ju |
Xylariales Xylariaceae |
Fungi | EU Protected Zone quarantine pest according to Commission Implementing Regulation (EU) 2019/2072 |
| 2 | Meloidogyne fallax | MELGFA | Meloidogyne fallax Karssen |
Rhabditida Meloidogynidae |
Nematodes | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072 |
| 3 | Phytophthora ramorum | PHYTRA | Phytophthora ramorum (non‐EU isolates) Werres, De Cock & Man in't Veld |
Peronosporales Peronosporaceae |
Oomycetes | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072 |
| 4 | Thaumetopoea processionea | THAUPR | Thaumetopoea processionea L. |
Lepidoptera Notodontidae |
Insects | EU Protected Zone quarantine pest according to Commission Implementing Regulation (EU) 2019/2072 |
5. RISK MITIGATION MEASURES
For each of the selected pests (Table 7), the Panel evaluated the likelihood that it could be present in the B. pendula and B. pubescens nurseries by evaluating the possibility that the commodity in the export nurseries is infested either by:
introduction of the pest from the environment surrounding the nursery;
introduction of the pest with new plants/seeds;
spread of the pest within the nursery.
The information used in the evaluation of the effectiveness of the risk mitigation measures is summarised in pest data sheets (see Appendix A).
5.1. Risk mitigation measures applied in the UK
With the information provided by the UK (Dossier Sections 1.1, 1.2, 2.0, 3.1, 3.2, 4.1, 4.2, 5.1, 5.2 and 5.3), the Panel summarised the risk mitigation measures (see Table 8) that are implemented in the production nursery.
TABLE 8.
Overview of implemented risk mitigation measures for Betula pendula and B. pubescens plants designated for export to the EU from the UK.
| Number | Risk mitigation measure | Implementation in the UK |
|---|---|---|
| 1 | Registration of production sites | All producers are registered as professional operators with the UK Competent Authority via APHA for England and Wales, or SASA for Scotland, and are authorised to issue the UK plant passports, verifying they meet the required national sanitary standards (Dossier Sections 1.1 and 1.2) |
| 2 | Physical separation | The majority of the nurseries also produce plants for the local market, and there is no distancing between production areas for the export and the local market. All plants within the UK nurseries are grown under the same phytosanitary measures, meeting the requirements of the UK Plant Passporting regime (Dossier Sections 1.1 and 1.2) |
| 3 | Certified plant material | Betula pendula and B. pubescens seeds purchased in the UK are certified under The Forest Reproductive Material (Great Britain) Regulations 2002 (legislation.gov.uk); seedlings sourced in the UK are certified with UK Plant Passports. A small percentage of seedlings may be obtained from EU (the Netherlands) and are certified with phytosanitary certificates (Dossier Sections 1.1 and 1.2) |
| 4 | Growing media | The growing media is virgin peat or peat‐free compost. This compost is heat‐treated by commercial suppliers during production to eliminate pests and diseases. It is supplied in sealed bulk bags or shrink‐wrapped bales and stored off the ground on pallets, these are free from contamination. Where delivered in bulk, compost is kept in a dedicated bunker, either indoors or covered by tarpaulin outdoors, and with no risk of contamination with soil or other material (Dossier Sections 1.1 and 1.2) |
| 5 | Surveillance, monitoring and sampling | For additional information see Section 3.3.3 Pest monitoring during production |
| 6 | Hygiene measures |
Growers must have an appropriate programme of weed management in place on the nursery (Dossier Sections 1.1 and 1.2) General hygiene measures are undertaken as part of routine nursery production, including disinfection of tools and equipment between batches/lots and different plant species. The tools are dipped in a disinfectant solution and wiped with a clean cloth between trees to reduce the risk of transfer of pests between subjects. There are various disinfectants available, with Virkon S (active substance: potassium peroxymonosulfate and sodium chloride) being a common example (Dossier Sections 1.1 and 1.2) |
| 7 | Removal of infested plant material | Post‐harvest and through the autumn and winter, nursery management is centred on pest and disease prevention and maintaining good levels of nursery hygiene. Leaves, pruning residues and weeds are all removed from the nursery to reduce the number of over wintering sites for pests and diseases (Dossier Sections 1.1 and 1.2) |
| 8 | Irrigation water | Water for irrigation is routinely sampled and sent for analysis (Dossier Sections 1.1 and 1.2) |
| 9 | Application of pest control measures |
Crop protection is achieved using a combination of measures including approved plant protection products, biological control or physical measures. Plant protection products are only used when necessary and records of all plant protection treatments are kept (Dossier Sections 1.1 and 1.2). Pest and disease pressure varies from season to season. Product application takes place only when required and depends on situation (disease pressure, growth stage etc. and environmental factors) at that time. Subject to this variation in pest pressure, in some seasons few, if any, pesticides are applied; in others it is sometimes necessary to apply preventative and/or control applications of pesticides. In many circumstances also, biological control is used to control outbreaks, rather than using chemical treatments (Dossier Sections 1.1 and 1.2). Examples of typical treatments used against aphids, caterpillars, rust fungi, spider mites and weeds are detailed in the Dossier Sections 1.1 and 1.2. These would be applied at the manufacturers recommended rate and intervals (Dossier Sections 1.1 and 1.2) |
| 10 | Measures against soil pests | There are no specific measures/treatments against the soil pests. However, containerised plants are grown in trays on top of protective plastic membranes to prevent contact with soil. Membranes are regularly refreshed when needed. Alternatively, plants may be grown on raised galvanised steel benches stood on gravel as a barrier between the soil and bench feet and/or concreted surfaces (Dossier Sections 1.1 and 1.2) |
| 11 | Inspections and management of plants before export |
The UK NPPO carries out inspections and testing where required by the country of destination's plant health legislation, to ensure all requirements are fulfilled and a valid phytosanitary certificate with the correct additional declarations is issued (Dossier Sections 1.1 and 1.2). Separate to any official inspection, plant material is checked by growers for plant health issues prior to dispatch (Dossier Sections 1.1 and 1.2). A final pre‐export inspection is undertaken as part of the process of issuing a phytosanitary certificate. These inspections are generally undertaken as near to the time of export as possible, usually within 1–2 days and not more than 2 weeks before export. Phytosanitary certificates are only issued if the commodity meets the required plant health standards after inspection and/or testing according to appropriate official procedures (Dossier Sections 1.1 and 1.2). The protocol for plants infested by pests during inspections before export is to treat the plants, if they are on site for a sufficient period of time or to destroy any plants infested by pests otherwise. All other host plants in the nursery would be treated. The phytosanitary certificate for export will not be issued until the UK Plant Health inspectors confirm that the plants are free from pests (Dossier Sections 1.1 and 1.2) |
| 12 | Separation during transport to the destination |
According to the Dossier Sections 1.1 and 1.2, the commodities are dispatched as single bare root trees or in bundles as follows:
Bare root plants are then wrapped in polythene and packed and distributed on ISPM 15 certified wooden pallets or metal pallets. Alternatively, they may be placed in pallets which are then wrapped in polythene. Small volume orders may be packed in waxed cardboard cartons or polythene bags and dispatched via courier (Dossier Sections 1.1 and 1.2). Rooted plants in pots are transported on Danish trolleys for smaller containers, or ISPM 15 certified pallets, or individually in pots for larger containers (Dossier Sections 1.1 and 1.2). Graftwood is wrapped in plastic and packed in cardboard boxes or Dutch crates on ISPM 15 certified wooden pallets or metal pallets, dependant on quantity (Dossier Sections 1.1 and 1.2). The preparation of the commodities for export is carried out inside the nurseries in a closed environment, e.g. packing shed, except for the specimen trees, which are prepared outside in an open field due to their dimensions (Dossier Sections 1.1 and 1.2). Plants are transported by lorry (size dependant on load quantity). Sensitive plants are occasionally transported by temperature‐controlled lorry if weather conditions during transit are likely to be very cold (Dossier Sections 1.1 and 1.2) |
5.2. Evaluation of the current measures for the selected relevant pests including uncertainties
For each evaluated pest, the relevant risk mitigation measures acting on the pest were identified. Any limiting factors on the effectiveness of the measures were documented.
All the relevant information including the related uncertainties deriving from the limiting factors used in the evaluation are summarised in a pest data sheet provided in Appendix A. Based on this information, for each selected relevant pest, an expert judgement is given for the likelihood of pest freedom taking into consideration the risk mitigation measures and their combination acting on the pest.
An overview of the evaluation of each relevant pest is given in the sections below (Sections 5.2.1, 5.2.2, 5.2.3–5.2.4). The outcome of the EKE regarding pest freedom after the evaluation of the currently proposed risk mitigation measures is summarised in Section 5.2.5.
5.2.1. Overview of the evaluation of Entoleuca mammata (Xylariales; Xylariaceae)
| Overview of the evaluation of E. mammata for graftwood/budwood | |||||
|---|---|---|---|---|---|
| Rating of the likelihood of pest freedom | Pest free with few exceptional cases (based on the median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free bundles | 9974 out of 10,000 bundles | 9985 out of 10,000 bundles | 9991 out of 10,000 bundles | 9995 out of 10,000 bundles | 9998.8 out of 10,000 bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected bundles | 1.2 out of 10,000 bundles | 5 out of 10,000 bundles | 9 out of 10,000 bundles | 15 out of 10,000 bundles | 26 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Entoleuca mammata is present in the UK, although not widely distributed. Betula pendula and B. pubescens are reported as hosts of the pathogen. Wounds could be present on twigs/branches taken for graftwood/budwood and may represent infection courts. The hosts can be present either inside or in the surroundings of the nurseries. Altogether, this suggests that association with the commodity may be possible. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material; (b) the removal of infected plant material and (c) application of plant protection products. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Betula plants for planting neither from the UK nor from other countries due to the presence of E. mammata between the years 1995 and January 2024 (EUROPHYT, 2024; TRACES‐NT, 2024). Shortcomings of current measures/procedures None observed
Main uncertainties
|
||||
| Overview of the evaluation of E. mammata for bare root plants | |||||
|---|---|---|---|---|---|
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants/bundles | 9927 out of 10,000 plants/bundles | 9961 out of 10,000 plants/bundles | 9979 out of 10,000 plants/bundles | 9991 out of 10,000 plants/bundles | 9998 out of 10,000 plants/bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants/bundles | 2 out of 10,000 plants/bundles | 9 out of 10,000 plants/bundles | 21 out of 10,000 plants/bundles | 39 out of 10,000 plants/bundles | 73 out of 10,000 plants/bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Because of the similarity of the commodities, the expected susceptibility to the pathogen and the production systems, and of the nurseries and surroundings, the Panel validated the scenarios from the previous Scientific opinion on Acer platanoides from the UK (EFSA PLH Panel, 2023a) for Betula pendula and B. pubescens. As a result of this evaluation, the same values as for Acer platanoides were considered to be applicable for B. pendula and B. pubescens. Entoleuca mammata is present in the UK, although not widely distributed. Betula pendula and B. pubescens are reported as hosts of the pathogen. Mechanical wounds including pruning wounds are expected to be present and may represent infection courts. The hosts can be present either inside or in the surroundings of the nurseries. Altogether, this suggests that association with the commodity may be possible. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material; (b) the removal of infected plant material and (c) application of plant protection products. |
||||
|
Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Betula plants for planting neither from the UK nor from other countries due to the presence of E. mammata between the years 1995 and January 2024 (EUROPHYT, 2024; TRACES‐NT, 2024). Shortcomings of current measures/procedures None observed
Main uncertainties
|
|||||
| Overview of the evaluation of E. mammata for plants in pots | |||||
|---|---|---|---|---|---|
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants/bundles | 9927 out of 10,000 plants/bundles | 9961 out of 10,000 plants/bundles | 9979 out of 10,000 plants/bundles | 9991 out of 10,000 plants/bundles | 9998 out of 10,000 plants/bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants/bundles | 2 out of 10,000 plants/bundles | 9 out of 10,000 plants/bundles | 21 out of 10,000 plants/bundles | 39 out of 10,000 plants/bundles | 73 out of 10,000 plants/bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Because of the similarity of the commodities, the expected susceptibility to the pathogen and the production systems, and of the nurseries and surroundings, the Panel validated the scenarios from the previous Scientific opinion on Acer platanoides from the UK (EFSA PLH Panel, 2023a) for B. pendula and B. pubescens. As a result of this evaluation, the same values as for A. platanoides were considered to be applicable for B. pendula and B. pubescens. E. mammata is present in the UK, although not widely distributed. B. pendula and B. pubescens are reported as hosts of the pathogen. Mechanical wounds including pruning wounds are expected to be present and may represent infection courts. The hosts can be present either inside or in the surroundings of the nurseries. Altogether, this suggests that association with the commodity may be possible. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material; (b) the removal of infected plant material and (c) application of plant protection products. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Betula plants for planting neither from the UK nor from other countries due to the presence of E. mammata between the years 1995 and January 2024 (EUROPHYT, 2024; TRACES‐NT, 2024). Shortcomings of current measures/procedures None observed
Main uncertainties
|
||||
| Overview of the evaluation of E. mammata for specimen trees | |||||
|---|---|---|---|---|---|
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9889 out of 10,000 plants | 9937 out of 10,000 plants | 9965 out of 10,000 plants | 9985 out of 10,000 plants | 9997 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 3 out of 10,000 plants | 15 out of 10,000 plants | 35 out of 10,000 plants | 63 out of 10,000 plants | 111 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Entoleuca mammata is present in the UK, although not widely distributed. Betula pendula and B. pubescens are reported as hosts of the pathogen. Mechanical wounds including pruning wounds are expected to be present in those specimen trees and may represent infection courts. The hosts can be present either inside or in the surroundings of the nurseries. Altogether, this suggests that association with the commodity may be possible. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material; (b) the removal of infected plant material and (c) application of plant protection products. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Betula plants for planting neither from the UK nor from other countries due to the presence of E. mammata between the years 1995 and January 2024 (EUROPHYT, 2024; TRACES‐NT, 2024). Shortcomings of current measures/procedures None observed
Main uncertainties
|
||||
5.2.2. Overview of the evaluation of Meloidogyne fallax (Rhabditida; Meloidogynidae)
| Overview of the evaluation of M. Fallax for bare root plants | |||||
|---|---|---|---|---|---|
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants/bundles | 9837 out of 10,000 plants/bundles | 9902 out of 10,000 plants/bundles | 9943 out of 10,000 plants/bundles | 9973 out of 10,000 plants/bundles | 9994 out of 10,000 plants/bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants/bundles | 6 out of 10,000 plants/bundles | 27 out of 10,000 plants/bundles | 57 out of 10,000 plants/bundles | 98 out of 10,000 plants/bundles | 163 out of 10,000 plants/bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The scenarios applied in the elicitation for Acer campestre in a previous EFSA opinion (EFSA PLH Panel, 2023b) were considered in the current elicitation. Meloidogyne fallax is present in the UK with restricted distribution. Suitable hosts are present in the surroundings. Betula pendula is a host of M. fallax. Due to the polyphagous nature of Meloidogyne spp. it is likely that also B. pubescens would be a host. The pest can enter the nurseries and spread within the nurseries with infected plant material and movement of soil attached to machinery, tools and shoes. The plants could become infected during the growth in the soil in the fields. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the nematode. These measures include (a) the use of certified plant material; (b) the use of heat‐treated growing media; (c) inspections, surveillance, monitoring, sampling and laboratory testing; and (d) hygiene measures. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Betula plants for planting neither from the UK nor from other countries due to the presence of M. fallax between the years 1995 and January 2024 (EUROPHYT, 2024; TRACES‐NT, 2024). Shortcomings of current measures/procedures Low‐pressure water is used for washing roots before export. This washing may not be as effective as using high pressure water in removing the soil, thereby making symptoms less visible.
Main uncertainties
|
||||
| Overview of the evaluation of M. fallax for plants in pots | |||||
|---|---|---|---|---|---|
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants/bundles | 9812 out of 10,000 plants/bundles | 9888 out of 10,000 plants/bundles | 9937 out of 10,000 plants/bundles | 9972 out of 10,000 plants/bundles | 9995 out of 10,000 plants/bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants/bundles | 5 out of 10,000 plants/bundles | 28 out of 10,000 plants/bundles | 63 out of 10,000 plants/bundles | 112 out of 10,000 plants/bundles | 188 out of 10,000 plants/bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The scenarios applied in the elicitation for Acer campestre in a previous EFSA opinion (EFSA PLH Panel, 2023b) were considered in the current elicitation. Meloidogyne fallax is present in the UK with restricted distribution. Suitable hosts are present in the surroundings of the nurseries. Betula pendula is a host of M. fallax. Due to the polyphagous nature of Meloidogyne spp. it is likely that also B. pubescens would be a host. The pest can enter the nurseries and spread within the nurseries with infected plant material and movement of soil attached to machinery, tools and shoes. The plants could become infected during the growth in the soil in the fields. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the nematode. These measures include (a) the use of certified plant material; (b) the use of heat‐treated growing media; (c) inspections, surveillance, monitoring, sampling and laboratory testing; (d) hygiene measures; and (e) separation of the pots from soil. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Betula plants for planting neither from the UK nor from other countries due to the presence of M. fallax between the years 1995 and January 2024 (EUROPHYT, 2024; TRACES‐NT, 2024). Shortcomings of current measures/procedures Inspections of plants in pots before export may not include root systems
Main uncertainties
|
||||
| Overview of the evaluation of M. fallax for specimen trees | |||||
|---|---|---|---|---|---|
| Rating of the likelihood of pest freedom | Very frequently pest free (based on the median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9735 out of 10,000 plants | 9824 out of 10,000 plants | 9895 out of 10,000 plants | 9952 out of 10,000 plants | 9991 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 9 out of 10,000 plants | 48 out of 10,000 plants | 105 out of 10,000 plants | 176 out of 10,000 plants | 265 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The scenarios applied in the elicitation for Acer campestre in a previous EFSA opinion (EFSA PLH Panel, 2023b) were considered in the current elicitation. Meloidogyne fallax is present in the UK with restricted distribution. Suitable hosts are present in the surroundings of the nurseries. Betula pendula is a host of M. fallax. Due to the polyphagous nature of Meloidogyne spp. it is likely that also B. pubescens would be a host. The pest can enter the nurseries and spread within the nurseries with infected plant material and movement of soil attached to machinery, tools and shoes. The plants could become infected during the growth in the soil in the fields. Contact with field soil may have been up to 9 years. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the nematode. These measures include (a) the use of certified plant material; (b) the use of heat‐treated growing media; (c) inspections, surveillance, monitoring, sampling and laboratory testing; and (d) hygiene measures. Interception records In the EUROPHYT/TRACES‐NT database, there are no records of notification of Betula plants for planting neither from the UK nor from other countries due to the presence of M. fallax between the years 1995 and January 2024 (EUROPHYT, 2024; TRACES‐NT, 2024). |
||||
| Summary of the information used for the evaluation |
Shortcomings of current measures/procedures None observed
Main uncertainties
|
||||
5.2.3. Overview of the evaluation of Phytophthora ramorum (non‐EU isolates) (Peronosporales; Peronosporaceae)
| Overview of the evaluation of P. ramorum (non‐EU isolates) for graftwood/budwood | |||||
|---|---|---|---|---|---|
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free bundles | 9964 out of 10,000 bundles | 9978 out of 10,000 bundles | 9988 out of 10,000 bundles | 9994 out of 10,000 bundles | 9998.8 out of 10,000 bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected bundles | 1.2 out of 10,000 bundles | 6 out of 10,000 bundles | 12 out of 10,000 bundles | 22 out of 10,000 bundles | 36 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Phytophthora ramorum is present in the UK with a restricted distribution. The pathogen has a wide host range including Betula pendula. The main hosts (e.g. Rhododendron spp., Larix spp. etc.) can be present either inside or in the surroundings of the nurseries. Aerial inoculum could be produced on these host plants and cause bark infections on the commodity. Measures taken against the pest and their efficacy Phytophthora ramorum is a quarantine pest in the UK and under official control. General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material and growing media; (b) inspections, surveillance, monitoring, sampling and laboratory testing; and (c) application of plant protection products. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Betula plants for planting neither from the UK nor from other countries due to the presence of P. ramorum between the years 1995 and January 2024 (EUROPHYT, 2024; TRACES‐NT, 2024). Shortcomings of current measures/procedures None observed
Main uncertainties
|
||||
| Overview of the evaluation of P. ramorum (non‐EU isolates) for bare root plants | |||||
|---|---|---|---|---|---|
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants/bundles | 9935 out of 10,000 plants/bundles | 9961 out of 10,000 plants/bundles | 9978 out of 10,000 plants/bundles | 9990 out of 10,000 plants/bundles | 9998 out of 10,000 plants/bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants/bundles | 2 out of 10,000 plants/bundles | 10 out of 10,000 plants/bundles | 22 out of 10,000 plants/bundles | 39 out of 10,000 plants/bundles | 65 out of 10,000 plants/bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Phytophthora ramorum is present in the UK with a restricted distribution. The pathogen has a wide host range including Betula pendula. The main hosts (e.g. Rhododendron spp., Larix spp. etc.) can be present either inside or in the surroundings of the nurseries. Aerial inoculum could be produced on these host plants and cause bark and leaf infections on the commodity. Measures taken against the pest and their efficacy Phytophthora ramorum is a quarantine pest in the UK and under official control. General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material and growing media; (b) inspections, surveillance, monitoring, sampling and laboratory testing; and (c) application of plant protection products. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Betula plants for planting neither from the UK nor from other countries due to the presence of P. ramorum between the years 1995 and January 2024 (EUROPHYT, 2024; TRACES‐NT, 2024). Shortcomings of current measures/procedures None observed
Main uncertainties
|
||||
| Overview of the evaluation of P. ramorum (non‐EU isolates) for plants in pots | |||||
|---|---|---|---|---|---|
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants/bundles | 9935 out of 10,000 plants/bundles | 9961 out of 10,000 plants/bundles | 9978 out of 10,000 plants/bundles | 9990 out of 10,000 plants/bundles | 9998 out of 10,000 plants/bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants/bundles | 2 out of 10,000 plants/bundles | 10 out of 10,000 plants/bundles | 22 out of 10,000 plants/bundles | 39 out of 10,000 plants/bundles | 65 out of 10,000 plants/bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Phytophthora ramorum is present in the UK with a restricted distribution. The pathogen has a wide host range including Betula pendula. The main hosts (e.g. Rhododendron spp., Larix spp. etc.) can be present either inside or in the surroundings of the nurseries. Aerial inoculum could be produced on these host plants and cause bark and leaf infections on the commodity. Measures taken against the pest and their efficacy P. ramorum is a quarantine pest in the UK and under official control. General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material and growing media; (b) inspections, surveillance, monitoring, sampling and laboratory testing; and (c) application of plant protection products. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Betula plants for planting neither from the UK nor from other countries due to the presence of P. ramorum between the years 1995 and January 2024 (EUROPHYT, 2024; TRACES‐NT, 2024). Shortcomings of current measures/procedures None observed
Main uncertainties
|
||||
| Overview of the evaluation of P. ramorum (non‐EU isolates) for specimen trees | |||||
|---|---|---|---|---|---|
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9915 out of 10,000 plants | 9946 out of 10,000 plants | 9969 out of 10,000 plants | 9986 out of 10,000 plants | 9997 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 3 out of 10,000 plants | 14 out of 10,000 plants | 31 out of 10,000 plants | 54 out of 10,000 plants | 85 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Phytophthora ramorum is present in the UK with a restricted distribution. The pathogen has a wide host range including Betula pendula. The main hosts (e.g. Rhododendron spp., Larix spp. etc.) can be present either inside or in the surroundings of the nurseries. Aerial inoculum could be produced on these host plants and cause bark and leaf infections on the commodity. Measures taken against the pest and their efficacy Phytophthora ramorum is a quarantine pest in the UK and under official control. General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material and growing media; (b) inspections, surveillance, monitoring, sampling and laboratory testing; and (c) application of plant protection products. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Betula plants for planting neither from the UK nor from other countries due to the presence of P. ramorum between the years 1995 and January 2024 (EUROPHYT, 2024; TRACES‐NT, 2024). Shortcomings of current measures/procedures None observed
Main uncertainties
|
||||
5.2.4. Overview of the evaluation of Thaumetopoea processionea (Lepidoptera; Notodontidae)
| Overview of the evaluation of T. processionea for bare root plants | |||||
|---|---|---|---|---|---|
| Rating of the likelihood of pest freedom | Almost always pest free (based on the median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants/bundles | 9991 out of 10,000 plants/bundles | 9995 out of 10,000 plants/bundles | 9997 out of 10,000 plants/bundles | 9999 out of 10,000 plants/bundles | 9999.86 out of 10,000 plants/bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants/bundles | 0.14 out of 10,000 plants/bundles | 1 out of 10,000 plants/bundles | 3 out of 10,000 plants/bundles | 5 out of 10,000 plants/bundles | 9 out of 10,000 plants/bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Because of the similarity of the commodities, the expected suitability to the pest, the production systems, the nurseries and surroundings, the Panel validated the scenarios from the previous Scientific opinion on Corylus avellana from the UK (EFSA PLH Panel, 2024) for Betula pendula and B. pubescens. As a result of this evaluation, the same values as for C. avellana were considered to be applicable for B. pendula and B. pubescens. Betula is not a reproductive host of T. processionea but if an outbreak is occurring in the nursery area on major hosts, some larvae can invade the Betula plants, moult into pupae that can be carried with them during transport. Measures taken against the pest and their efficacy Plants are surveyed and larvae should be detected as at that stage they are large and conspicuous because of the long whitish hairs. The Panel assumes that infested plants will be removed. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Betula plants for planting neither from the UK nor from other countries due to the presence of T. processionea between the years 1995 and January 2024 (EUROPHYT, 2024; TRACES‐NT, 2024). Shortcomings of current measures/procedures None observed
Main uncertainties
|
||||
| Overview of the evaluation of T. processionea for plants in pots | |||||
|---|---|---|---|---|---|
| Rating of the likelihood of pest freedom | Almost always pest free (based on the median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants/bundles | 9991 out of 10,000 plants/bundles | 9995 out of 10,000 plants/bundles | 9997 out of 10,000 plants/ bundles |
9999 out of 10,000 plants/ bundles |
9999.86 out of 10,000 plants/ bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants/bundles |
0.14 out of 10,000 plants/ bundles |
1 out of 10,000 plants/ bundles |
3 out of 10,000 plants/ bundles |
5 out of 10,000 plants/ bundles |
9 out of 10,000 plants/ bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Because of the similarity with regard to the suitability of the commodity for T. processionea the same values were taken as for bare root plants. Betula is not a reproductive host of T. processionea but if an outbreak is occurring in the nursery area on oaks, some larvae can invade the Betula plants and ultimately moult into pupae. Both can be carried with the plants during transport, as plants can be traded with leaves. Measures taken against the pest and their efficacy Plants are surveyed and larvae should be detected as at that stage they are large and conspicuous because of the long whitish hairs. The Panel assumes that infested plants will be removed. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Betula plants for planting neither from the UK nor from other countries due to the presence of T. processionea between the years 1995 and January 2024 (EUROPHYT, 2024; TRACES‐NT, 2024). Shortcomings of current measures/procedures None observed
Main uncertainties
|
||||
| Overview of the evaluation of T. processionea for specimen trees | |||||
|---|---|---|---|---|---|
| Rating of the likelihood of pest freedom | Pest free with few exceptional cases (based on the median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9981 out of 10,000 plants | 9989 out of 10,000 plants | 9993 out of 10,000 plants | 9996 out of 10,000 plants | 9998.9 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 1.1 out of 10,000 plants | 4 out of 10,000 plants | 7 out of 10,000 plants | 11 out of 10,000 plants | 19 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Betula is not a reproductive host of T. processionea but if an outbreak is occurring in the nursery area on major hosts, some larvae can invade the Betula plants and ultimately moult into pupae. Both can be carried with the plants during transport, as plants can be traded with leaves. Measures taken against the pest and their efficacy Plants are surveyed and larvae should be detected as at that stage they are large and conspicuous because of the long whitish hairs. The Panel assumes that infested plants will be removed. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Betula plants for planting neither from the UK nor from other countries due to the presence of T. processionea between the years 1995 and January 2024 (EUROPHYT, 2024; TRACES‐NT, 2024). Shortcomings of current measures/procedures The precision of the survey done in the nursery when preparing the plants for delivery, as larvae can be hidden in the canopy especially on large trees. |
||||
|
Main uncertainties
|
|||||
5.2.5. Outcome of Expert Knowledge Elicitation
Table 9 and Figure 4 show the outcome of the EKE regarding pest freedom after the evaluation of the implemented risk mitigation measures for all the evaluated pests.
TABLE 9.
Assessment of the likelihood of pest freedom following evaluation of current risk mitigation measures against pests on Betula pendula and B. pubescens plants designated for export to the EU.
| Number | Group | Pest species | Sometimes pest free | More often than not pest free | Frequently pest free | Very frequently pest free | Extremely frequently pest free | Pest free with some exceptional cases | Pest free with few exceptional cases | Almost always pest free |
|---|---|---|---|---|---|---|---|---|---|---|
| Commodity 1: bundles of graftwood and budwood | ||||||||||
| 1 | Fungi | Entoleuca mammata | L | M | U | |||||
| 2 | Oomycetes | Phytophthora ramorum (non‐EU isolates) | LM | U | ||||||
| Commodity 2: bare root plants (bundles of whips and transplants and single bare root plants) | ||||||||||
| 3 | Fungi | Entoleuca mammata | L | M | U | |||||
| 4 | Nematodes | Meloidogyne fallax | L | M | U | |||||
| 5 | Oomycetes | Phytophthora ramorum (non‐EU isolates) | L | M | U | |||||
| 6 | Insects | Thaumetopoea processionea | L | MU | ||||||
| Commodity 3: plants in pots (bundles of cell‐grown plants and single plants in pots) | ||||||||||
| 7 | Fungi | Entoleuca mammata | L | M | U | |||||
| 8 | Nematodes | Meloidogyne fallax | L | M | U | |||||
| 9 | Oomycetes | Phytophthora ramorum (non‐EU isolates) | L | M | U | |||||
| 10 | Insects | Thaumetopoea processionea | L | MU | ||||||
| Commodity 4: single specimen trees | ||||||||||
| 11 | Fungi | Entoleuca mammata | L | M | U | |||||
| 12 | Nematodes | Meloidogyne fallax | LM | U | ||||||
| 13 | Oomycetes | Phytophthora ramorum (non‐EU isolates) | L | M | U | |||||
| 14 | Insects | Thaumetopoea processionea | L | M | U | |||||
FIGURE 4.
Elicited certainty (y‐axis) of the number of pest‐free plants/bundles of Betula pendula and B. pubescens (x‐axis; log‐scaled) out of 10,000 plants/bundles designated for export to the EU from the UK for all evaluated pests visualised as descending distribution function. Horizontal llines indicate the reported certainty levels (starting from the bottom 5%, 25%, 50%, 75%, 95%) Please see the reading instructions below.
Figure 5 provides an explanation of the descending distribution function describing the likelihood of pest freedom after the evaluation of the implemented risk mitigation measures for Betula pendula and B. pubescens specimen trees designated for export to the EU for Meloidogyne fallax.
FIGURE 5.
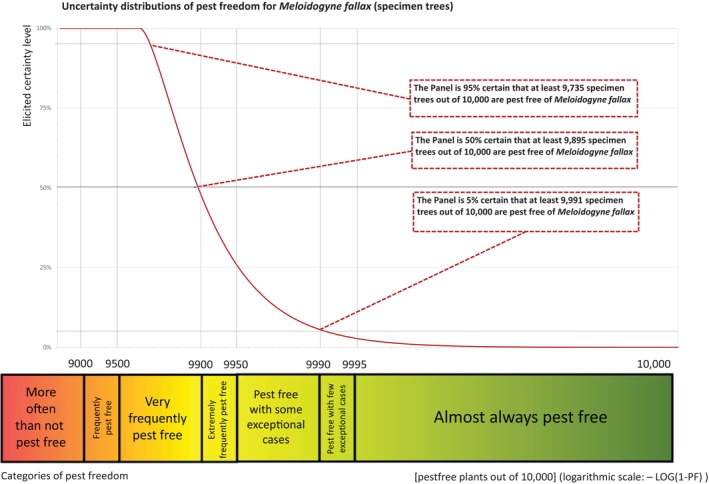
Explanation of the descending distribution function describing the likelihood of pest freedom after the evaluation of the implemented risk mitigation measures for plants designated for export to the EU based on based on the example of Meloidogyne fallax on Betula pendula and B. pubescens specimen trees.
PANEL A PANEL B
| Pest‐freedom category | Pest‐free plants/bundles out of 10,000 | Legend of pest‐freedom categories | |||
|---|---|---|---|---|---|
| Sometimes pest free | ≤ 5000 | L | Pest freedom category includes the elicited lower bound of the 90% uncertainty range | ||
| More often than not pest free | 5000–≤ 9000 | M | Pest‐freedom category includes the elicited median | ||
| Frequently pest free | 9000–≤9500 | U | Pest‐freedom category includes the elicited upper bound of the 90% uncertainty range | ||
| Very frequently pest free | 9500–≤ 9900 | ||||
| Extremely frequently pest free | 9900–≤ 9950 | ||||
| Pest free with some exceptional cases | 9950–≤ 9990 | ||||
| Pest free with few exceptional cases | 9990–≤ 9995 | ||||
| Almost always pest free | 9995–≤ 10,000 | ||||
Notes: In panel A, the median value for the assessed level of pest freedom for each pest is indicated by ‘M', the 5% percentile is indicated by ‘L' and the 95% percentile is indicated by ‘U'. The percentiles together span the 90% uncertainty range regarding pest freedom. The pest‐freedom categories are defined in panels A and B of the table.
6. CONCLUSIONS
There are four pests identified to be present in the UK and considered to be potentially associated with the commodities imported from the UK and relevant for the EU.
These pests are Entoleuca mammata, Meloidogyne fallax, Phytophthora ramorum (non‐EU isolates) and Thaumetopoea processionea. The likelihood of pest freedom after the evaluation of the implemented risk mitigation measures for the commodities designated for export to the EU was estimated. In the assessment of risk, the age of the plants was considered, reasoning that older trees are more likely to be infested mainly due to longer exposure time and larger size making inspection more difficult.
The category ‘bare root plants’ includes the commodities 1‐to 2‐year‐old whips (bundles of 5–15 plants) and transplants (bundles of 5–50 plants) and 1‐to 7‐year‐old single bare root plants. The category ‘plants in pots’ includes the commodities 1‐to 2‐year‐old cell‐grown plants in bundles and 1‐to 7‐year‐old single plants in pots. The commodities graftwood/budwood and large specimen trees were evaluated as single categories.
The commodity graftwood/budwood is not expected to be infected/infested by M. fallax and T. processionea.
For E. mammata the likelihood of pest freedom for bundles of graftwood and budwood following evaluation of current risk mitigation measures was estimated as ‘pest free with few exceptional cases’ with the 90% uncertainty range reaching from ‘pest free with some exceptional cases’ to ‘almost always pest free’. The EKE indicated, with 95% certainty, that between 9974 and 10,000 bundles of graftwood and budwood per 10,000 will be free from E. mammata. The likelihood of pest freedom for bare root plants and plants in pots was identical because of similarities in the suitability to the pathogen and detection probability. For these two commodity categories, the likelihood was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘extremely frequently pest free’ to ‘almost always pest free’. The EKE indicated, with 95% certainty, that between 9927 and 10,000 bare root plants and plants in pots per 10,000 will be free from E. mammata. The likelihood of pest freedom for specimen trees was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘almost always pest free’. The EKE indicated, with 95% certainty, that between 9889 and 10,000 specimen trees up per 10,000 will be free from E. mammata.
For M. fallax the likelihood of pest freedom for bare root plants was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases’. The EKE indicated, with 95% certainty, that between 9837 and 10,000 bare root plants per 10,000 will be free from M. fallax. The likelihood of pest freedom for plants in pots was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases. The EKE indicated, with 95% certainty, that between 9812 and 10,000 plants in pots up per 10,000 will be free from M. fallax. The likelihood of pest freedom for specimen trees was estimated as ‘very frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases'. The EKE indicated, with 95% certainty, that between 9735 and 10,000 specimen trees up per 10,000 will be free from M. fallax.
For P. ramorum (non‐EU isolates) the likelihood of pest freedom for bundles of graftwood and budwood following evaluation of current risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range reaching from ‘pest free with some exceptional cases’ to ‘almost always pest free’. The EKE indicated, with 95% certainty, that between 9964 and 10,000 bundles of graftwood and budwood per 10,000 will be free from P. ramorum (non‐EU isolates). The likelihood of pest freedom for bare root plants and plants in pots was identical because of similarities in the suitability to the pathogen and detection probability. For these two categories, the likelihood of pest freedom for bare root plants was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘extremely frequently pest free’ to ‘almost always pest free’. The EKE indicated, with 95% certainty, that between 9935 and 10,000 bare root plants and plants in pots per 10,000 will be free from P. ramorum (non‐EU isolates). The likelihood of pest freedom for specimen trees was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘extremely frequently pest free’ to ‘almost always pest free’. The EKE indicated, with 95% certainty, that between 9915 and 10,000 specimen trees up per 10,000 will be free from P. ramorum (non‐EU isolates).
For T. processionea, the likelihood of pest freedom for bare root plants and plants in pots was identical because of similarities in the suitability to the insect and detection probability. For these two categories, the likelihood of pest freedom for bare root plants and plants in pots was estimated as ‘almost always pest free’ with the 90% uncertainty range spanning from ‘pest free with few exceptional cases’ to ‘almost always pest free’. The EKE indicated, with 95% certainty, that between 9991 and 10,000 bare root plants per 10,000 will be free from T. processionea. The likelihood of pest freedom for specimen trees was estimated as ‘pest free with few exceptional cases’ with the 90% uncertainty range spanning from ‘pest free with some exceptional cases’ to ‘almost always pest free’. The EKE indicated, with 95% certainty, that between 9981 and 10,000 specimen trees up per 10,000 will be free from T. processionea.
GLOSSARY
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 2024a, 2024b).
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2024b).
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2024b).
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units.
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2024b).
- Measures
Control (of a pest) is defined in ISPM 5 (FAO, 2024b) as ‘Suppression, containment or eradication of a pest population’ (FAO, 2024a). Control measures are measures that have a direct effect on pest abundance. Supporting measures are organisational measures or procedures supporting the choice of appropriate risk mitigation measures that do not directly affect pest abundance.
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2024b).
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2024b).
- Protected zone
A Protected zone is an area recognised at EU level to be free from a harmful organism, which is established in one or more other parts of the Union.
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2024b).
- Regulated non‐quarantine pest
A non‐quarantine pest whose presence in plants for planting affects the intended use of those plants with an economically unacceptable impact and which is therefore regulated within the territory of the importing contracting party (FAO, 2024b).
- Risk mitigation measure
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A risk mitigation measure may become a phytosanitary measure, action or procedure according to the decision of the risk manager.
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2024b).
ABBREVIATIONS
- APHA
Animal and Plant Health Agency
- CABI
Centre for Agriculture and Bioscience International
- DEFRA
Department for Environment Food and Rural Affairs
- EFSA
European Food Safety Authority
- EKE
Expert Knowledge Elicitation
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- ISPM
International Standards for Phytosanitary Measures
- NPPO
National Plant Protection Organisation
- PHSI
Plant Health and Seeds Inspectorate
- PLH
Plant Health
- PRA
Pest Risk Assessment
- RNQPs
Regulated Non‐Quarantine Pests
- SASA
Science and Advice for Scottish Agriculture
CONFLICT OF INTEREST
If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
REQUESTOR
European Commission
QUESTION NUMBER
EFSA‐Q‐2023‐00508; EFSA‐Q‐2023‐00510
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
MAP DISCLAIMER
The designations employed and the presentation of material on any maps included in this scientific output do not imply the expression of any opinion whatsoever on the part of the European Food Safety Authority concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
PANEL MEMBERS
Antonio Vicent Civera, Paula Baptista, Anna Berlin, Elisavet Chatzivassiliou, Jaime Cubero, Nicholas Cunniffe, Eduardo De La Peña, Nicolas Desneux, Francesco Di Serio, Anna Filipiak, Paolo Gonthier, Beata Hasiow‐Jaroszewska, Hervé Jactel, Blanca B. Landa, Lara Maistrello, David Makovski, Panagiotis Milonas, Nikos T. Papadopulos, Roel Potting, Hanna Susi, and Dirk Jan Van Der Gaag.
Supporting information
Excel file with the pest list of Betula pendula and B. pubescens
ACKNOWLEDGEMENTS
The Scientific Opinion was prepared in cooperation with the Universita degli studi di Padova, Dipartimento Agronomia, Animali, Alimenti, Risorse Naturali e Ambiente (Italy) under the EFSA Art. 36 Framework Partnership Agreement ‘GP/EFSA/PLANTS/2022/11’ commodity risk assessment for forestry plants.
APPENDIX A. Data sheets of pests selected for further evaluation
A.1. Entoleuca mammata
A.1.1. Organism information
| Taxonomic information |
Current valid scientific name: Entoleuca mammata Synonyms: Anthostoma blakei, Anthostoma morsei, Fuckelia morsei, Hypoxylon blakei, Hypoxylon holwayi, Hypoxylon mammatum, Hypoxylon morsei, Hypoxylon pauperatum, Hypoxylon pruinatum, Nemania mammata, Rosellinia pruinata, Sphaeria mammata, Sphaeria pruinata (according to Index Fungorum, 2024) Name used in the EU legislation: Entoleuca mammata (Wahlenb.) Rogers and Ju Order: Xylariales Family: Xylariaceae Common name: Hypoxylon canker of poplar, canker of poplar, canker of aspen Name used in the Dossier: Entoleuca mammata Note: For an extensive review on taxonomy of the genera Nemania, Hypoxylon and Entoleuca, see Granmo et al. (1999) |
|
| Group | Fungi | |
| EPPO code | HYPOMA | |
| Regulated status |
Entoleuca mammata is listed in Annex III of Commission Implementing Regulation (EU) 2019/2072 as protected zone quarantine pest for Ireland. The pathogen is quarantine pest in China and Israel. It is on the A1 list of Türkiye (EPPO, 2024a). |
|
| Pest status in the UK | E. mammata is present in the UK, with few occurrences in England, Wales, Channel Islands and Scotland (CABI, 2019; EPPO, 2024b; Mathiassen, 1993). | |
| Pest status in the EU | E. mammata is reported from the following EU MS: Austria, Belgium, Croatia, Czechia, Finland, France, Germany, Greece, Italy, Lithuania, the Netherlands, Slovakia, Slovenia, Sweden (EFSA PLH Panel, 2017), Denmark (GBIF, 2024), Estonia (Lutter et al., 2019), Latvia (Zeps et al., 2016); Poland and Spain (Farr & Rossman, 2024). | |
| Host status on Betula pendula and Betula pubescens |
E. mammata was reported on Betula pubescens in Finland (Granmo et al., 1999) and on Betula pendula (Betula alba) in Sweden (Mathiassen, 1993). E. mammata is reported as a pathogen of Betula alleghaniensis (synonym: Betula lutea), Betula papyrifera (Conners, 1967; Ginns, 1986) and Betula sp. (EPPO, 2024c; Ginns, 1986). |
|
| PRA information | Pest Risk Assessments available:
|
|
| Other relevant information for the assessment | ||
| Biology |
E. mammata is an ascomycete fungus mostly known as pathogen causing canker disease in Populus tremuloides and P. tremula (EFSA PLH Panel, 2017), as well as primary saprophyte on several Salix species (Mathiassen, 1993). The fungus was firstly described as Sphaeria mammata on Betula alba (current name: B. pendula) from Swedish Lapland in 1826 (Mathiassen, 1993), but it is thought to be native to North America and introduced into Europe several centuries ago (Kasanen et al., 2004). E. mammata is now largely distributed in the temperate zones of the northern hemisphere; it is present in Canada and in several states of the USA (CABI, 2019; EPPO, 2024b). In Asia, E. mammata is only found in the Korea Republic on decayed wood (Lee et al., 2000). In Europe, in addition to the mentioned EU MS and the UK (see above), it is reported from Andorra, Russia, Serbia, Switzerland, Ukraine (CABI, 2019; EPPO, 2024b) and Norway (Granmo et al., 1999; NBIC, 2021). The presence of E. mammata in Australia is uncertain (few specimens in herbarium without other records) (EPPO, 2024b). The ascospores of E. mammata infect the living wood penetrating in the periderm and invading tissues under bark through mechanical wounds and injuries caused by woodpeckers and insects (Anderson et al., 1979a; Ostry & Anderson, 1983); water stress can increase host susceptibility (EFSA PLH Panel, 2017). E. mammata overwinters in host tissues both as mycelium and spores. Five to 14 months after infection conidia are produced, but their role in the disease transmission is not relevant (EFSA PLH Panel, 2017). The pathogen is mostly found on trees 15–40 years‐old, but all ages can be infected (EFSA PLH Panel, 2017; EPPO 2024d). Infection usually starts from branches and twigs and then spreads to the main stem. E. mammata is most frequently found on stems about 1.5–2.5 m above the ground (Mathiassen, 1993). The cankers expand very rapidly (7–8 cm per month) in summer, and more slowly during winter; branches and stems can be girdled causing drying and breakage. The fungus mostly develops in the range from 8°C to 32°C, the optimum temperature is 28°C; toxins host‐specific produced by the fungus are involved in pathogenesis (EFSA PLH Panel, 2017; EPPO, 2023; Stermer et al., 1984). E. mammata can spread over long distances via windborne ascospores, which are produced 2–3 years after infection; cankers on felled trees on the ground continue to produce ascospores for 23 months. Ascospores are dispersed with a temperature above −4°C and wet weather; a minimum of 16°C is required for starting germination, which became rapid at 28–32°C (EFSA PLH Panel, 2017). Infected wood, mostly with bark, may be a pathway for passive spread of E. mammata in international trade; however, also young plants may carry ascospores or mycelium of the fungus, which can survive as a latent infection on living material inadvertently moved (EFSA PLH Panel, 2017; EPPO, 2023). E. mammata is an important pathogen of poplars in the USA and Canada, causing economic losses of millions of dollars a year (Anderson et al., 1979b; EFSA PLH Panel, 2017; Ostry, 2013). In Europe E. mammata is known as a pest of low importance, although damage on Populus tremula has been reported in France (Pinon, 1976) and Italy (EFSA PLH Panel, 2017) and in poplar plantations in Sweden and Estonia (EFSA PLH Panel, 2017; Lutter et al., 2019). Data on the incidence and impact of E. mammata on woody species other than poplars and willows are poor or absent, and may be considered negligible; on Betula, the fungus only occurs on ‘very deteriorated wood’ (Granmo et al., 1999). |
|
| Symptoms | Main type of symptoms |
There is no information on the symptoms caused to Betula plants. However, the symptoms are generic and they are described for Populus trees. Early symptoms of cankers on the bark appear as slightly sunken, yellowish‐orange areas with an irregular border. Young cankers can be identified by removing the bark to expose the white mycelium in the cambial zone. The outer bark in older cankers is then lifted into blister‐like patches and break away, exposing blackened areas prominently visible on green branches and trunks. Callus formation only occasionally develops because cankers spread very quickly (Anderson et al., 1979b; EPPO, 2023). Wilting of leaves may be observed when living trees are girdled by cankers, as well as sprouting of new shoots on stem and branches. Infected trees can be secondarily colonised by other fungi, accelerating the host decline (EPPO, 2023). |
| Presence of asymptomatic plants | On poplar, the disease caused by E. mammata has a latent period and symptoms can appear only 2 years after the ascospore infection, therefore asymptomatic plants can be found (Ostry & Anderson, 2009). | |
| Confusion with other pests |
Some Hypoxylon species present in Europe on deciduous trees (H. confluens and H. udum) show symptoms similar to those of E. mammata but can be easily distinguished in laboratory by the ascospore characteristics (EFSA PLH Panel, 2017). According to Granmo et al. (1999), E. mammata is also easily distinguished from species of Nemania by its oligoperitheciate erumpent stromata and polygonal perithecial demarcations. |
|
| Host plant range |
In North America, E. mammata mainly infects P. tremuloides. Minor damage is recorded on P. alleghaniensis, P. balsamifera, P. grandidentata and various Populus hybrids. Other secondary hosts in North America are Acer, Alnus, Betula, Carpinus, Fagus, Picea, Pyrus, Salix, Sorbus and Ulmus (Manion & Griffin, 1986). In Europe, the main hosts are poplars, mostly P. tremula. Other hosts are P. alba, P. nigra, P. trichocarpa and the hybrid P. tremula × P. tremuloides (Ostry, 2013). However, in the central and northern Scandinava willows seem to be the main hosts of E. mammata, mostly Salix caprea, S. pentandra and S. myrsinifolia. The fungus is here also found on Populus and Sorbus, whereas Betula is considered only a secondary host (Mathiassen, 1993). In the long list of specimens examined by Granmo et al. (1999) just one record of Betula pubescens as host of E. mammata is reported. In the UK, E. mammata has been reported on Salix in Wales (Mathiassen, 1993). Betula alleghaniensis (synonym: Betula lutea), Betula papyrifera are also hosts of E. mammata according to Conners (1967), Ginns (1986) and Granmo et al. (1999). |
|
| Reported evidence of impact | E. mammata is an EU protected zone quarantine pest. | |
| Evidence that the commodity is a pathway |
Plants for planting may carry ascospores and mycelium of E. mammata also asymptomatically (EFSA PLH Panel, 2017; EPPO 2024d), therefore the commodity is a pathway. E. mammata is believed to have been introduced at least once in the last century into France with plant material (flowering branches of Populus tremula) used for hybridisation (EPPO, 2024d). |
|
| Surveillance information | E. mammata is not a regulated pest for the UK and it is not under official control and surveillance. However, Great Britain exports to Northern Ireland are required to be free from E. mammata to ensure Northern Ireland remains a pest free protected zone (Dossier Section 5.1). | |
A.1.2. Possibility of pest presence in the nursery
A.1.2.1. Possibility of entry from the surrounding environment
E. mammata is present in the UK in England, Wales, Channel Islands and Scotland (CABI, 2019; EPPO, 2024b; Mathiassen, 1993).
The pathogen can easily spread with ascospores dispersed by air currents also over long distance.
E. mammata can infect Acer spp., Alnus spp., Betula alleghaniensis, B. papyrifera, B. lenta, Quercus robur and Populus spp., Populus tremuloides, which are present within 2 km from the nurseries in woodlands and hedgerows. Other possible hosts, as Betula and Salix might be present in the private gardens in the same area (Dossier Sections 1.1, 1.2 and 5.1).
Uncertainties
-
–
The presence of the pathogen in the surrounding area.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nurseries from surrounding environment via ascospores transported by wind and air currents.
A.1.2.2. Possibility of entry with new plants/seeds
The starting materials are either seeds, seedlings or shoots/buds when grafted plants are produced. Seeds are certified and coming from the UK. Seedlings are either from the UK and the EU (mostly the Netherlands) (Dossier Sections 1.1 and 1.2).
In addition to Betula pendula and B. pubescens plants, the nurseries also produce other plants (Dossier Sections 3.1 and 3.2). Out of them, there are suitable hosts for the pathogen such as Acer spp., Alnus spp., Carpinus spp., Fagus spp., Malus spp., Picea spp., Populus nigra and P. tremula, Pyrus spp., Quercus robur, Salix spp., Sorbus aucuparia and Ulmus spp. However, there is no information on how and where the plants are produced. Therefore, if the plants are first produced in another nursery, the pathogen could possibly travel with them.
The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Sections 1.1 and 1.2). The growing media is certified and heat‐treated by commercial suppliers during production to eliminate pests and diseases. There is no evidence that soil or growing media may be a pathway for E. mammata.
Uncertainties
-
–
No information is available on the provenance of new plants other than Betula used for plant production in the nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nurseries via new seedlings of Betula and plants of other species used for plant production in the area. The entry of the pathogen with seeds and the growing media the Panel considers as not possible.
A.1.2.3. Possibility of spread within the nursery
B. pendula and B. pubescens plants are either grown in containers (cells, pots, tubes, etc.) outdoors, in the open air or in field. Cell‐grown trees may be grown in greenhouses, however most plants will be field grown or field grown in containers (Dossier Sections 1.1 and 1.2). Mother plants of B. pendula are present in one of the nurseries, from which shoots are taken for grafting (Dossier Sections 1.1 and 1.2). Adult trees 15–40 years‐old are more susceptible to be infected by E. mammata (EFSA PLH Panel, 2017); moreover, mechanical wounds are a way of entry for the pathogen, and the close association between sharp wounds and cankers is known (EPPO, 2023).
The pathogen can infect other suitable plants present in the nurseries, such as Acer spp., Alnus spp., Carpinus spp., Fagus spp., Malus spp., Picea spp., Populus nigra and P. tremula etc. present within the nurseries (Dossier Sections 3.1 and 3.2).
Once entered, ascospores of E. mammata could be produced on infected plants and naturally spread within the nurseries by air currents.
Uncertainties
-
–
Whether ascospores are produced on infected nursery plants.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pathogen within the nurseries is possible by air currents as well as via shoots used for grafting taken from infected mother plants.
A.1.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database there are no records of notification of Betula plants for planting neither from the UK nor from other countries due to the presence of E. mammata between the years 1995 and January 2024 (EUROPHYT, 2024; TRACES‐NT, 2024).
A.1.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on E. mammata is provided. The description of the risk mitigation measures currently applied in the UK is provided in the Table 8.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
The risk mitigation measure is expected to be effective in reducing the likelihood of the presence of the pathogen on the commodity.
Uncertainties
|
| 2 | Physical separation | No | Not relevant |
| 3 | Certified plant material | Yes |
The risk mitigation measure is expected to be effective in reducing the likelihood of the presence of the pathogen on the commodity.
Uncertainties
|
| 4 | Growing media | No | Not relevant |
| 5 | Surveillance, monitoring and sampling | Yes |
Entoleuca mammata is not a regulated pest for the UK and it is not under official control and surveillance. However, Great Britain exports to Northern Ireland are required to be free from E. mammata to ensure Northern Ireland remains a pest free protected zone.
Uncertainties
|
| 6 | Hygiene measures | No | Not relevant |
| 7 | Removal of infested plant material | Yes |
This measure could have some effect.
Uncertainties
|
| 8 | Irrigation water | No | Not relevant |
| 9 | Application of pest control measures | Yes |
Although E. mammata is generally not a target of the pesticide treatments in the nurseries, some fungicides could reduce the likelihood of the infection by the pathogen.
Uncertainties
|
| 10 | Measures against soil pests | No | Not relevant |
| 11 | Inspections and management of plants before export | Yes |
This measure could have some effect.
Uncertainties
|
| 12 | Separation during transport to the destination | No | Not relevant |
A.1.5. Overall likelihood of pest freedom for graftwood/budwood
A.1.5.1. Reasoning for a scenario which would lead to a reasonably low number of infected graftwood/budwood
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Younger woody tissues are exposed to the pathogen for only short period of time. The scenario assumes Betula spp. to be unsuitable/minor hosts for the pathogen. Graftwood/budwood is taken in winter, when infectious inoculum may be absent. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.1.5.2. Reasoning for a scenario which would lead to a reasonably high number of infected graftwood/budwood
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. Young woody tissues are susceptible to the pathogen. The scenario assumes Betula spp. to be relatively suitable hosts for the pathogen. Graftwood/budwood is taken when infectious inoculum is present. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.1.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected graftwood/budwood (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings and that the plants are exposed to the pathogen for a sufficient period of time to cause infection through mechanical wounds. The scenario also assumes that graftwood/budwood is taken in winter when no infectious inoculum is present. No wounds are expected to be widespread on graftwood/budwood (with the exception of those originated from cutting). Betula spp. are considered minor hosts.
A.1.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pathogen in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.1.5.5. Elicitation outcomes of the assessment of the pest freedom for Entoleuca mammata on graftwood/budwood
The following Tables show the elicited and fitted values for pest infection (Table A.1) and pest freedom (Table A.2).
TABLE A.1.
Elicited and fitted values of the uncertainty distribution of pest infection by Entoleuca mammata per 10,000 bundles of graftwood/budwood.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0.0 | 4.5 | 9.0 | 15.0 | 35.0 | ||||||||||
| EKE results | 0.371 | 0.732 | 1.24 | 2.12 | 3.22 | 4.54 | 5.90 | 8.91 | 12.7 | 15.1 | 18.2 | 21.9 | 26.4 | 30.4 | 35.0 |
Note: The EKE results is the BetaGeneral (1.3743, 7.4777, 0, 69) distribution fitted with @Risk version 7.6.
Based on the numbers of estimated infected bundles the pest freedom was calculated (i.e. = 10,000 – number of infected bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.2.
TABLE A.2.
The uncertainty distribution of plants free of Entoleuca mammata per 10,000 bundles of graftwood/budwood calculated by Table A.1.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 9965.0 | 9985.0 | 9991.0 | 9995.5 | 10,000.0 | ||||||||||
| EKE results | 9965 | 9970 | 9974 | 9978 | 9982 | 9985 | 9987 | 9991 | 9994 | 9995 | 9996.8 | 9997.9 | 9998.8 | 9999.3 | 9999.6 |
Note: The EKE results are the fitted values.
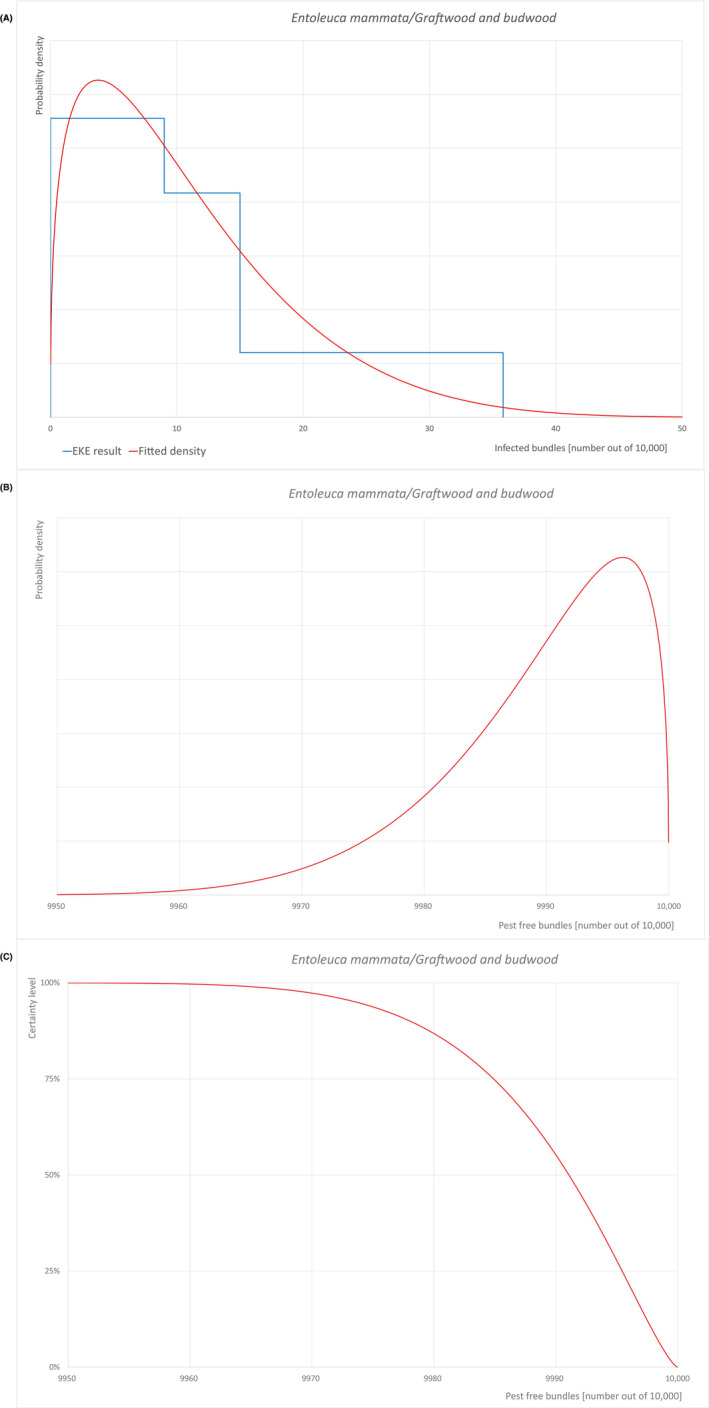
FIGURE A.1 (A) Elicited uncertainty of pest infection per 10,000 bundles of graftwood/budwood (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (B) uncertainty of the proportion of pest‐free bundles per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (C) descending uncertainty distribution function of pest infection per 10,000 bundles.
A.1.6. Overall likelihood of pest freedom for bare root plants
The scenarios as well as the values were taken from the Scientific opinion on Acer platanoides from the UK (EFSA PLH Panel, 2023) because of the similarity of the commodities, in their susceptibility to the pathogen, of the production systems and of the nurseries and surroundings.
A.1.6.1. Reasoning for a scenario which would lead to a reasonably low number of infected bare root plants
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Younger plants are exposed to the pathogen for only short period of time. The scenario assumes Betula spp. to be unsuitable/minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.1.6.2. Reasoning for a scenario which would lead to a reasonably high number of infected bare root plants
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. Older plants are exposed to the pathogen for longer period of time. The scenario assumes Betula spp. to be hosts for the pathogen. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.1.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bare root plants (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings and that the plants are exposed to the pathogen for a sufficient period of time to cause infection through mechanical wounds. Betula spp. are considered minor hosts.
A.1.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pathogen in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.1.6.5. Elicitation outcomes of the assessment of the pest freedom for Entoleuca mammata on bare root plants
The following Tables show the elicited and fitted values for pest infection (Table A.3) and pest freedom (Table A.4).
TABLE A.3.
Elicited and fitted values of the uncertainty distribution of pest infection by Entoleuca mammata per 10,000 plants/bundles of bare root plants.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 10 | 20 | 40 | 100 | ||||||||||
| EKE results | 0.418 | 0.987 | 1.90 | 3.72 | 6.20 | 9.44 | 12.9 | 21.1 | 31.8 | 38.9 | 48.4 | 59.5 | 73.3 | 85.6 | 100 |
Note: The EKE results is the BetaGeneral (1.0764, 6.8505, 0, 200) distribution fitted with @Risk version 7.6.
Based on the numbers of estimated infected bundles the pest freedom was calculated (i.e. = 10,000 – number of infected plants/bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.4.
TABLE A.4.
The uncertainty distribution of plants free of Entoleuca mammata per 10,000 plants/bundles of bare root plants calculated by Table A.3.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eliciteed values | 9900 | 9960 | 9980 | 9990 | 10,000 | ||||||||||
| EKE results | 9900 | 9914 | 9927 | 9940 | 9952 | 9961 | 9968 | 9979 | 9987 | 9991 | 9994 | 9996 | 9998 | 9999.0 | 9999.6 |
Note: The EKE results are the fitted values.
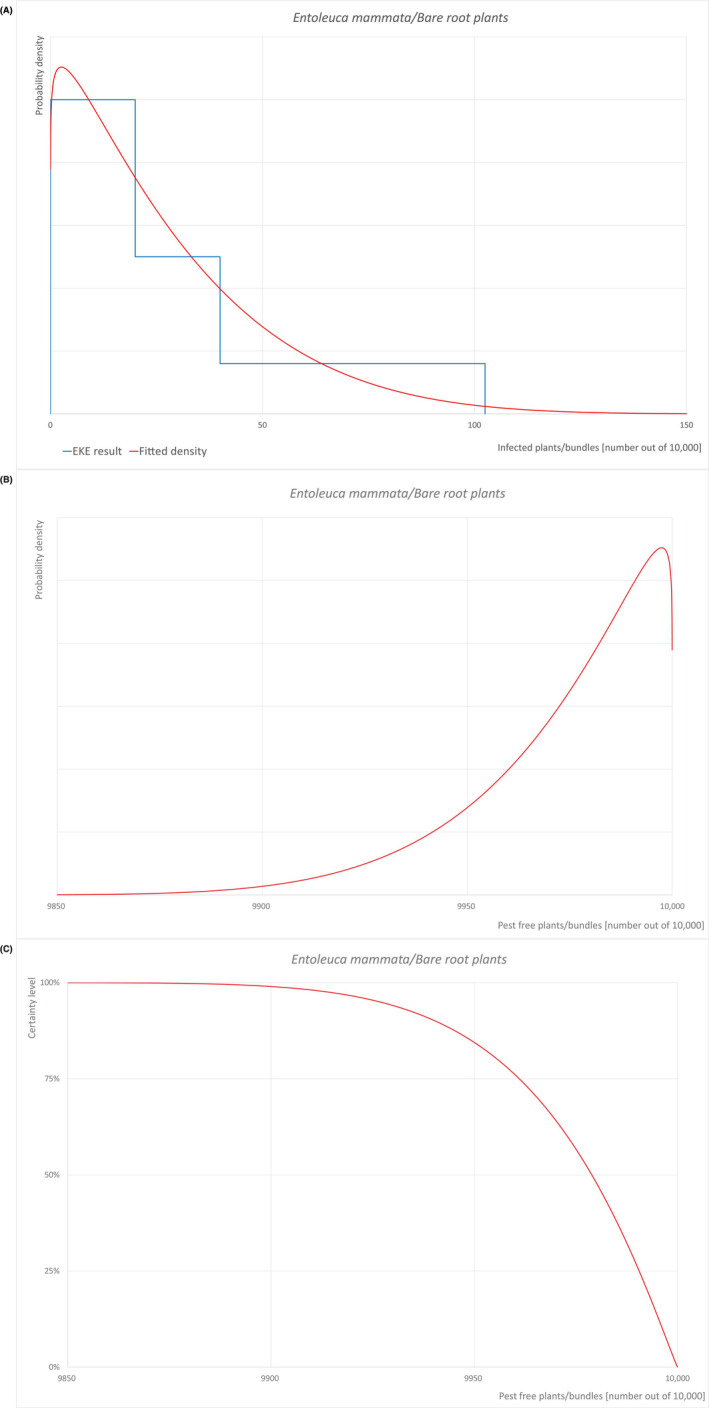
FIGURE A.2 (A) Elicited uncertainty of pest infection per 10,000 plants/bundles of bare root plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (B) uncertainty of the proportion of pest‐free plants/bundles per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (C) descending uncertainty distribution function of pest infection per 10,000 plants/bundles.
A.1.7. Overall likelihood of pest freedom for plants in pots
The scenarios as well as the values were taken from the Scientific opinion on Acer platanoides from the UK (EFSA PLH Panel, 2023) because of the similarity of the commodities, in their susceptibility to the pathogen, of the production systems and of the nurseries and surroundings.
A.1.7.1. Reasoning for a scenario which would lead to a reasonably low number of infected plants in pots
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Younger plants are exposed to the pathogen for only short period of time. The scenario assumes Betula spp. to be unsuitable/minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.1.7.2. Reasoning for a scenario which would lead to a reasonably high number of infected plants in pots
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. Older plants are exposed to the pathogen for longer period of time. The scenario assumes Betula spp. to be hosts for the pathogen. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.1.7.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected plants in pots (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings and that the plants are exposed to the pathogen for a sufficient period of time to cause infection through mechanical wounds. Betula spp. are considered minor hosts.
A.1.7.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pathogen in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.1.7.5. Elicitation outcomes of the assessment of the pest freedom for Entoleuca mammata on plants in pots
The following Tables show the elicited and fitted values for pest infection (Table A.5) and pest freedom (Table A.6).
TABLE A.5.
Elicited and fitted values of the uncertainty distribution of pest infection by Entoleuca mammata per 10,000 plants/bundles of plants in pots.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 10 | 20 | 40 | 100 | ||||||||||
| EKE results | 0.418 | 0.987 | 1.90 | 3.72 | 6.20 | 9.44 | 12.9 | 21.1 | 31.8 | 38.9 | 48.4 | 59.5 | 73.3 | 85.6 | 100 |
Note: The EKE results is the BetaGeneral (1.0764, 6.8505, 0, 200) distribution fitted with @Risk version 7.6.
Based on the numbers of estimated infected bundles the pest freedom was calculated (i.e. = 10,000 – number of infected plants/bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.6.
TABLE A.6.
The uncertainty distribution of plants free of Entoleuca mammata per 10,000 plants/bundles of plants in pots calculated by Table A.5.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 9900 | 9960 | 9980 | 9990 | 10,000 | ||||||||||
| EKE results | 9900 | 9914 | 9927 | 9940 | 9952 | 9961 | 9968 | 9979 | 9987 | 9991 | 9994 | 9996 | 9998 | 9999.0 | 9999.6 |
Note: The EKE results are the fitted values.
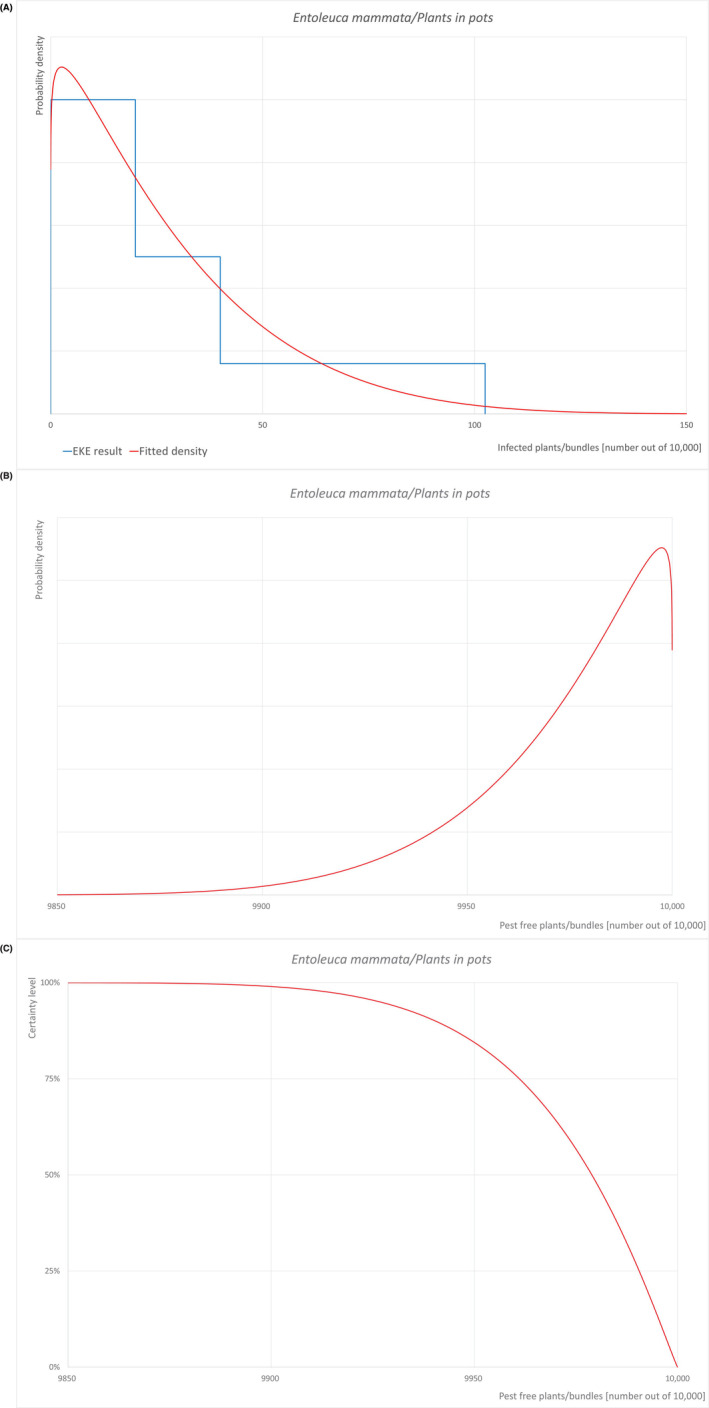
FIGURE A.3 (A) Elicited uncertainty of pest infection per 10,000 plants/bundles of plants in pots (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (B) uncertainty of the proportion of pest‐free plants/bundles per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (C) descending uncertainty distribution function of pest infection per 10,000 plants/bundles.
A.1.8. Overall likelihood of pest freedom for specimen trees
A.1.8.1. Reasoning for a scenario which would lead to a reasonably low number of infected specimen trees
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. The scenario assumes Betula spp. to be unsuitable/minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.1.8.2. Reasoning for a scenario which would lead to a reasonably high number of infected specimen trees
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. Older plants are exposed to the pathogen for longer period of time. Several pruning has been carried out on those specimen trees providing infection courts. The scenario assumes Betula spp. to be hosts for the pathogen. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections because trees are big, and symptoms can be hidden by the foliage.
A.1.8.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected specimen trees (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings and that the plants are exposed to the pathogen long enough to cause infection through mechanical wounds, including pruning wounds. Betula spp. are considered minor hosts.
A.1.8.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pathogen in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.1.8.5. Elicitation outcomes of the assessment of the pest freedom for Entoleuca mammata on specimen trees
The following Tables show the elicited and fitted values for pest infection (Table A.7) and pest freedom (Table A.8).
TABLE A.7.
Elicited and fitted values of the uncertainty distribution of pest infection by Entoleuca mammata per 10,000 specimen trees.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0.0 | 16.5 | 33.0 | 65.0 | 140.0 | ||||||||||
| EKEresults | 0.579 | 1.44 | 2.89 | 5.86 | 10.0 | 15.5 | 21.3 | 34.9 | 52.2 | 63.3 | 77.3 | 92.9 | 111 | 125 | 140 |
Note: The EKE results is the BetaGeneral (1.0099, 3.4532, 0, 190) distribution fitted with @Risk version 7.6.
Based on the numbers of estimated infected bundles the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.8.
TABLE A.8.
The uncertainty distribution of plants free of Entoleuca mammata per 10,000 specimen trees calculated by Table A.7.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 9860.0 | 9935.0 | 9967.0 | 9983.5 | 10,000.0 | ||||||||||
| EKE results | 9860 | 9875 | 9889 | 9907 | 9923 | 9937 | 9948 | 9965 | 9979 | 9985 | 9990 | 9994 | 9997 | 9998.6 | 9999.4 |
Note: The EKE results are the fitted values.
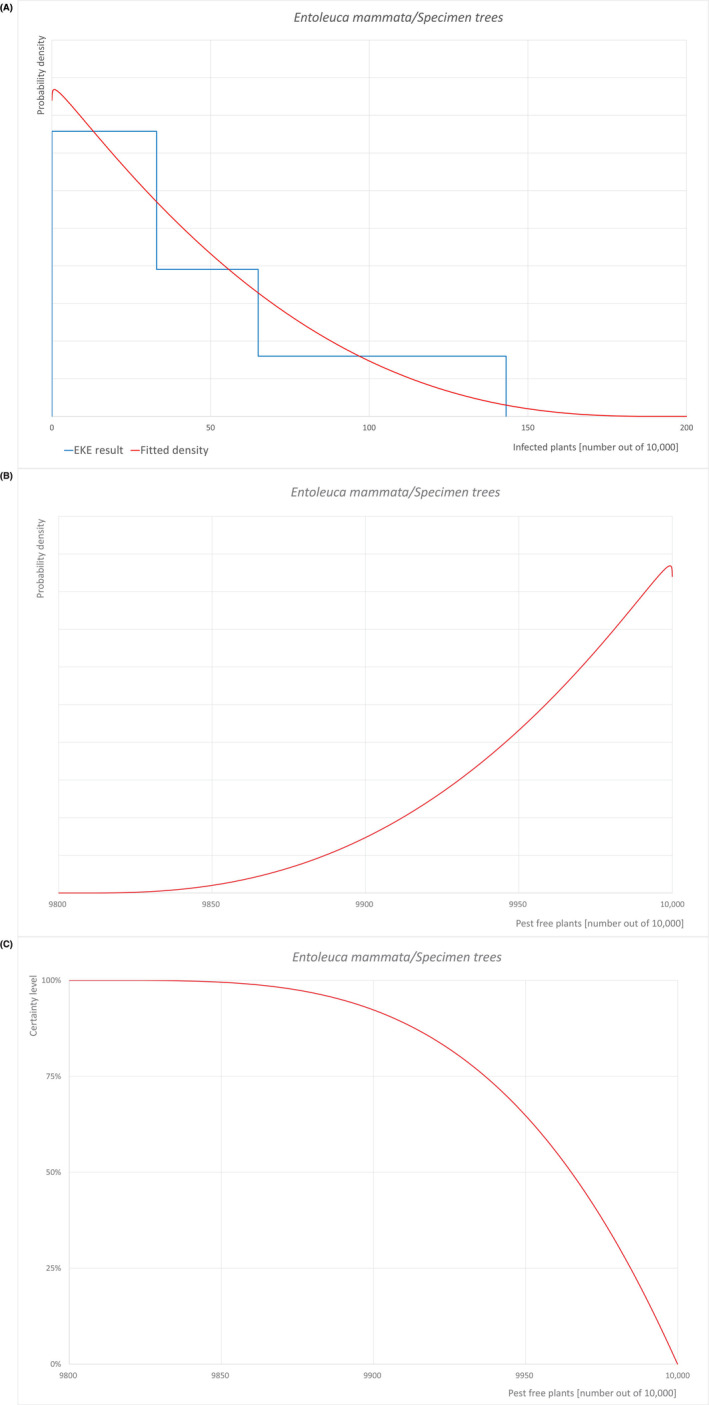
FIGURE A.4 (A) Elicited uncertainty of pest infection per 10,000 plants of specimen trees (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (B) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (C) descending uncertainty distribution function of pest infection per 10,000 plants.
A.1.9. Reference list
Anderson, N. A., Ostry, M. E., & Anderson, G. W. (1979a). Insect wounds as infection sites for Hypoxylon mammatum on trembling aspen. Phytopathology, 69, 476–479. https://doi.org/10.1094/phyto‐69‐476
Anderson, R. L., Anderson, G. W., & Schipper, A. L. Jr. (1979b). Hypoxylon canker of aspen. USDA Forest Insect and Disease Leaflet, 6, 6 pp.
CABI (Centre for Agriculture and Bioscience International). (2019). Hypoxylon mammatum (poplar canker). https://www.cabi.org/cpc/datasheet/28323 (accessed 2024‐02‐04).
Conners, I. L. (1967). An annotated index of plant diseases in Canada and fungi recorded on plants in Alaska, Canada and Greenland. Research Branch Canada Department of Agriculture, 1251, 1–381.
DEFRA (Department for Environment, Food and Rural Affairs). (2023). UK risk register details for Entoleuca mammata. https://planthealthportal.defra.gov.uk/pests‐and‐diseases/uk‐plant‐health‐risk‐register/viewPestRisks.cfm?cslref=11840 (accessed 2024‐02‐05).
EFSA PLH Panel (EFSA Panel on Plant Health), Jeger, M., Bragard, C., Caffier, D., Candresse, T., Chatzivassiliou, E., Dehnen Schmutz, K., Gilioli, G., Gregoire, J‐C., Jaques Miret, J. A., MacLeod, A., Navajas Navarro, M., Niere, B., Parnell, S., Potting, R., Rafoss, T., Rossi, V., Urek, G., Van Bruggen, A., Van der Werf, W., West, J., Winter, S., Boberg, J., Gonthier, P., & Pautasso, M. (2017). Scientific Opinion on the pest categorisation of Entoleuca mammata. EFSA Journal, 15(7), 4925. https://doi.org/10.2903/j.efsa.2017.4925
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard, C., Baptista, P., Chatzivassiliou, E., Di Serio, F., Jaques Miret, J. A., Justesen, A. F., MacLeod, A., Magnusson, C. S., Milonas, P., Navas‐Cortes, J. A., Parnell, S., Potting, R., Reignault, P. L., Stefani, E., Thulke, H.‐H., Van der Werf, W., Vicent Civera, A., Yuen, J., Zappalà, L., Battisti, A., Mas, H., Rigling, D., Faccoli, M., Gardi, C., Iacopetti, G., Mikulová, A., Mosbach‐Schulz, O., Stergulc, F., Streissl, F., & Gonthier, P. (2023). Scientific Opinion on the commodity risk assessment of Acer platanoides plants from the UK. EFSA Journal, 21(7), 8073. https://doi.org/10.2903/j.efsa.2023.8073
EPPO (European and Mediterranean Plant Protection Organization). (2023). Entoleuca mammata (HYPOMA), Datasheet. https://gd.eppo.int/taxon/HYPOMA/datasheet (accessed 2024‐02‐04).
EPPO (European and Mediterranean Plant Protection Organization). (2024a). Entoleuca mammata (HYPOMA), Categorization. https://gd.eppo.int/taxon/HYPOMA/categorization (accessed 2024‐02‐04).
EPPO (European and Mediterranean Plant Protection Organization). (2024b). Entoleuca mammata (HYPOMA), Distribution. https://gd.eppo.int/taxon/HYPOMA/distribution (accessed 2024‐02‐04).
EPPO (European and Mediterranean Plant Protection Organization). (2024c). Entoleuca mammata (HYPOMA), Hosts. https://gd.eppo.int/taxon/HYPOMA/hosts (accessed 2024‐02‐04).
EPPO (European and Mediterranean Plant Protection Organization). (2024d). Outbreak in France of Aspen Canker caused by Hypoxylon mammatum (Wahl) Mill. (= H. pruinatum (Klotz) Cke). https://gd.eppo.int/reporting/article‐5822 (accessed 2024‐02‐08).
EUROPHYT (European Union Notification System for Plant Health Interceptions). (2024). https://food.ec.europa.eu/plants/plant‐health‐and‐biosecurity/europhyt_en (accessed 2024‐02‐09).
Farr, D. F., & Rossman, A. Y. (2024). Fungal Databases, U.S. National Fungus Collections, ARS, USDA. https://fungi.ars.usda.gov/ (accessed 2024‐02‐09).
GBIF (Global Biodiversity Information Facility) Secretariat. (2024). GBIF BackBone Taxonomy. https://www.gbif.org/ (accessed 2024‐02‐04).
Ginns, J.H. (1986). Compendium of plant disease and decay fungi in Canada 1960–1980. Biosystematics Research Center, Ottawa, Ontario, 1813, 416 pp.
Granmo, A., Laessoe, T., & Schumacher, T. (1999). The genus Nemania s.l. (Xylariaceae) in Norden. Sommerfeltia, 27, 1–96.
Index Fungorum. (2024). https://www.indexfungorum.org/. (accessed 2024‐02‐04)
Kasanen, R., Hantula, J., Ostry, M. E., Pinon, J., & Kurkela, T. (2004). North American populations of Entoleuca mammata are genetically more variable than populations in Europe. Mycological Research, 108, 766–774. https://doi.org/10.1017/s0953756204000334
Klejdysz, T., Kubasik, W., Strażyński, P., Gawlak, M., Pruciak, A., Rzepecka, D., & Kałuski, T. (2018). Express pest risk analysis for Hypoxylon mammatum. https://www.plantquarantine.pl/pl/?node_id=1683&literka=E (accessed 2024‐02‐05).
Lee, Y. S., Han, S. S., Shin, J. H., Lee, Y. M., & Song, B. K. (2000). Germ tube formation of ascospores of two terrestrial higher ascomycetes, Hypoxylon mammatum and H. truncatum. Journal of Korean Wood Science and Technology, 28, 10–16.
Lutter, R., Drenkhan, R., Tullus, A., Jürimaa, K., Tullus, T., & Tullus, H. (2019). First record of Entoleuca mammata in hybrid aspen plantations in hemiboreal Estonia and stand‐environmental factors affecting its prevalence. European Journal of Forest Research, 138(2), 263–274. https://doi.org/10.1007/s10342‐019‐01165‐7
Manion, P. D., & Griffin, D. H. (1986). Sixty‐five years of research on Hypoxylon canker of aspen. Plant Disease, 70(8), 803–808. https://doi.org/10.1094/pd‐70‐803
Mathiassen, G. (1993). Corticolous and lignicolous Pyrenomycetes s. lat. (Ascomycetes) on Salix along a mid‐Scandinavian transect. Sommerfeltia, 20, 1–180.
NBIC (Norwegian Biodiversity Information Center). (2021). Entoleuca mammata. https://artsdatabanken.no/Taxon/Entoleuca%20mammata/82864
Ostry, M. E. (2013). Hypoxylon canker. In P. Gonthier & G. Nicolotti (Eds.), Infectious Forest Diseases (pp. 407–419). CABI International, Wallingford.
Ostry, M. E., & Anderson, N. A. (1983). Infection of trembling aspen by Hypoxylon mammatum through cicada oviposition wounds. Phytopathology, 73, 1092–1096. https://doi.org/10.1094/phyto‐73‐1092
Ostry, M. E., & Anderson, N. A. (2009). Genetics and ecology of the Entoleuca mammata–Populus pathosystem: implications for aspen improvement and management. Forest Ecology and Management, 257, 390–400. https://doi.org/10.1016/j.foreco.2008.09.053
Stermer, B. A., Scheffer, R. P., & Hart, J. H. (1984). Isolation of toxins from Hypoxylon mammatum and demonstration of some toxin effects on selected clones of Populus tremuloides. Phytopathology, 74, 654–658. https://doi.org/10.1094/phyto‐74‐654
TRACES‐NT. (2024). TRAde Control and Expert System. https://webgate.ec.europa.eu/tracesnt (accessed 2024‐02‐09).
Zeps, M., Adamovics, A., Smilga, J., & Sisenis, L. (2016). Productivity and quality of hybrid aspen at the age of 18 years. Research for Rural Development, 2, 55–61.
A.2. Meloidogyne fallax
A.2.1. Organism information
| Taxonomic information |
Current valid scientific name: Meloidogyne fallax Synonyms: Meloidogyne chitwoodi B‐type Name used in the EU legislation: Meloidogyne fallax Karssen [MELGFA] Order: Rhabditida Family: Meloidogynidae Common name: False Columbia root‐knot nematode, root gall nematode, root‐knot nematode Name used in the Dossier: Meloidogyne fallax |
|
| Group | Nematodes | |
| EPPO code | MELGMA | |
| Regulated status |
The pest is listed in Annex II of Regulation (EU) 2019/2072 as Meloidogyne fallax Karssen [MELGFA]. The pest is included in the EPPO A2 list (EPPO, 2024a). Meloidogyne fallax is quarantine in Morocco, Moldova and Norway. It is on A1 list of Argentina, Bahrain, Brazil, Egypt, Georgia, Kazakhstan, Russia, Ukraine and EAEU (=Eurasian Economic Union – Armenia, Belarus, Kazakhstan, Kyrgyzstan and Russia). It is on A2 list of COSAVE (=Comite de Sanidad Vegetal del Cono Sur – Argentina, Brazil, Chile, Paraguay, Peru and Uruguay) (EPPO, 2024b). Meloidogyne fallax is also quarantine pest in the USA (Kantor et al., 2022). In the UK M. fallax is a regulated non‐quarantine pest in Great Britain on potato only, as this is considered to be the main host at risk (DEFRA, 2024; EPPO, 2024b; James et al., 2019) and it is a regulated quarantine pest in Northern Ireland (DEFRA, 2024). |
|
| Pest status in the UK |
M. fallax is present in the UK (CABI, 2021; EPPO, 2024c) with restricted distribution and no findings associated to trees. The pest status of M. fallax in the UK is officially declared as: present, restricted distribution – under containment, in case eradication is impossible (EPPO 2024d). The nematode was first recorded in the UK in 2011 in sports turf and in 2013 in a leek crop in Staffordshire. In 2015 it has been newly recorded from sports turf in NW England and in 2018 in a carrots field in East Anglia (EPPO, 2015, 2024d; Everatt et al., 2016; James et al., 2019). The presence of M. fallax in Northern Ireland (EPPO, 2015) is no longer confirmed as it was due to a mistake (EPPO, 2024d). |
|
| Pest status in the EU |
M. fallax is present in Belgium, France, Germany (transient), the Netherlands and Sweden (present, under eradication) (EPPO, 2024c, 2024d). M. fallax has been found in Ireland in the past century (1965) (Topalović et al., 2017), but it has not been reported since. |
|
| Host status on Betula pendula and B. pubescens |
Betula pendula is reported as a host plant for M. fallax in field experiments (den Nijs et al., 2004). No information on B. pendula and B. pubescens as hosts of M. fallax in natural conditions was found. |
|
| PRA information | Available Pest Risk Assessments:
|
|
| Other relevant information for the assessment | ||
| Biology |
M. fallax is a highly polyphagous root‐knot nematode firstly described from the Netherlands and distributed in temperate regions of the world mostly in agricultural/horticultural crops (Everatt et al., 2016). M. fallax has been found in a natural habitat in the Netherlands in 2023 (EPPO, 2024e). It is present in Africa (South Africa), Asia (Indonesia), Europe (Belgium, France, Germany, the Netherlands, Switzerland, Sweden, the UK), Oceania (Australia, New Zealand), South America (Chile) (CABI, 2021; EPPO, 2024c). According to MacLeod et al. (2012) M. fallax may be more widespread because it is frequently confused with similar species as M. hapla and M. chitwoodi, and not causing clear external symptoms on host plants. M. fallax has six development stages: eggs, juveniles (four stages) and adults. The nematode mainly reproduces parthenogenetically, and sexual reproduction can possibly occur under adverse conditions; like other Meloidogyne species, M. fallax has one to three generations per year depending on temperature and host availability (EFSA, 2019; MacLeod et al., 2012). Females lay up to 800–1000 eggs in gelatinous masses on the root surface, in galls and tubers. Hatching can occur at temperatures below 10°C, so that M. fallax is considered cryophilic (EFSA PLH Panel, 2020; MacLeod et al., 2012). The second‐stage juveniles move in the soil and penetrate host roots, start feeding on cortical tissues inducing the formation of root galls; they become sedentary and develop to successive stages by quick moults. The nematode can stay infective in the soil for long time, being also able to survive for more than 300 days at temperatures of 5 and 10°C, and 140 days at higher temperatures (15–25°C). Survival and infectivity may also be related to high soil humidity (100% survival with 98% RH) although in moderate dry soil conditions M. fallax may survive for more than 9 weeks (MacLeod et al., 2012). Similar to other nematode species living in the soil, M. fallax has only little spread capacity, the juvenile stages moving 1–2 m maximum per year depending on type of soil, water availability and other parameters (EFSA, 2019). Water could also disperse the nematode (mainly eggs and juveniles) at short distances. The human‐assisted spread on medium‐long distance is very frequent and effective by passive transport. Possible pathways are plants for planting with infected roots; tubers and bulbs; soil and growing media; contaminated tools, machinery, shoes and packaging material (EFSA, 2019). It is believed that outbreaks of M. fallax in the UK in leek crops and sports turf are due to introduction with infected plant waste, soil and machinery (James et al., 2019). M. fallax is known as a species of economic concern on some horticultural crops as potato and carrot, mostly in the Netherlands, but no information is available on yield losses. The main damage observed is the reduction of merchantability in potato tubers (MacLeod et al., 2012). Similarly, no significant damage was observed on strawberries (Van der Sommen et al., 2005). In the UK, reduced growth of leek plants was reported in an organic crop in Staffordshire (EPPO, 2024d). Damage caused by M. fallax in sports turf were reported in North‐western England in 2015 (EPPO, 2015; Everatt et al., 2016). No specific data about damage on B. pendula or Betula sp. was found. |
|
| Symptoms | Main type of symptoms |
M. fallax is a root‐knot nematode. Heavily infested plants show stunting and yellowing on above‐ground parts and galling on roots (EFSA, 2019; MacLeod et al., 2012; Moens et al., 2009). Symptoms of root‐knot nematodes on hardwood trees may show as slow growth, sparse foliage, chlorotic leaves and crown dieback (Riffle, 1963). Symptoms on roots vary with species but should be visible as galls in advanced infections. On potato tubers, M. fallax cause brown point‐like necroses just under the skin developing into numerous small pimple‐like areas (tuber galls) on the surface (CABI, 2021; EPPO, 2019). No specific information about symptoms on B. pendula or Betula sp. was found. |
| Presence of asymptomatic plants | At the early stages of infection, plants may not show any apparent symptoms on the above‐ground parts and do not show galls on the roots. In some cases, plants are wilted and lack vigour. The main impact of the pest is on root growth, and on the quality and growth of the plant (EFSA, 2019; Moens et al., 2009; MacLeod et al., 2012). | |
| Confusion with other pests |
M. fallax is morphologically very similar to M. chitwoodi and may also be easily confused with other species as M. hapla and M. minor, often found in the same habitat. M. fallax cannot be identified on the basis of sole galls, since other soil nematode cause similar damage and some insects and bacteria can induce comparable galls on roots as well (EFSA, 2019). The nematode can be identified by laboratory tests on morphometric characters, electrophoresis or sequencing /DNA barcoding are needed (EPPO, 2016). |
|
| Host plant range |
M. fallax is a polyphagous nematode with a wide host range, including several major horticultural and agricultural crops and a few species of trees, shrubs and herbaceous plants. Main horticultural/agricultural hosts are: Apium graveolens, Allium porrum, Asparagus officinalis, Avena strigosa, Beta vulgaris, Cicorium endivia, Cynara scolymus, Daucus carota, Foeniculum vulgare, Fragaria ananassa, Hordeum vulgare, Lactuca sativa, Lycopersicum esculentum, Medicago sativa, Phaseolus vulgaris, Secale cereale, Solanum nigrum, S. tuberosum, Solanum spp., Triticum aestivum and Zea mays (CABI, 2021; EPPO, 2024f; MacLeod et al., 2012). Woody hosts of M. fallax are Acer palmatum, Betula pendula, Cornus sanguinea, Laburnum anagyroides, Lonicera xylosteum (Ferris, 2024; MacLeod et al., 2012). For a more exhaustive list of hosts see CABI (2021), EPPO (2024f), Ferris (2024), den Nijs et al. (2004), MacLeod et al. (2012). |
|
| Reported evidence of impact | M. fallax is an EU quarantine pest. | |
| Evidence that the commodity is a pathway |
Meloidogyne nematodes, although rarely identified at species level, are frequently intercepted on plants for planting, for example Acer palmatum, Cryptomeria sp., Diospyros kaki, Ficus sp. Fraxinus sp., Juniperus chinensis, Ligustrum sp., Punica granatum, Taxus cuspidata, Zelkova sp. (EUROPHYT, 2024; TRACES‐NT, 2024). B. pendula is a host plant of M. fallax; therefore, the commodity is a possible pathway of entry for the nematode. |
|
| Surveillance information | M. fallax is a pest not currently meeting the criteria of quarantine pest for the UK (see Regulated status). It is considered under official control only in limited outbreak areas (EPPO, 2024d). M. fallax is not included in the pest list of the Dossier, and no specific surveillance protocols are currently expected. | |
A.2.2. Possibility of pest presence in the nursery
A.2.2.1. Possibility of entry from the surrounding environment
Meloidogyne fallax is present in the UK territory with restricted distribution in agricultural lands and sports turf (EPPO, 2024c, 2024d; James et al., 2019).
The nematode has limited capacity of movement in the soil (1–2 m) and can only spread by passive transport human assisted with plants for planting with infected roots, infected soil and growing media, and possibly via contaminated tools and machinery. No other possibility of entry in the nurseries is known.
M. fallax can infect Allium porrum, Beta vulgaris, Daucus carota, Hordeum vulgare, Lactuca sativa, Lolium spp., Lolium multiflorum, Medicago sativa, Solanum tuberosum, Triticum spp., Zea mays, which are present in arable crops and pastures within 2 km from the nurseries (Dossier Sections 1.1, 1.2 and 5.1).
Uncertainties
-
–
None.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the nematode to enter the nurseries from surrounding environment. In the surrounding area, suitable hosts are present, but the nematode cannot enter by other way than human assisted spread.
A.2.2.2. Possibility of entry with new plants/seed
The starting materials are either seeds, seedlings or shoots/buds when grafted plants are produced. Seeds are certified and coming from the UK. Seedlings are either from the UK and the EU (mostly the Netherlands) (Dossier Sections 1.1 and 1.2). Seeds and shoots/buds are not a pathway for the nematode.
In addition to B. pendula and B. pubescens, the nurseries also produce other plants (Dossier Sections 3.1 and 3.2). Out of them, there are some suitable hosts for the nematode (such as Acer palmatum, Cornus sanguinea, Laburnum anagyroides and Lonicera xylosteum). However, there is no information on how and where the plants are produced. Besides, M. fallax may also spread on soil adhering to the roots of non‐host plants (MacLeod et al., 2012). Therefore, if the plants are first produced in another nursery, the nematode could possibly travel with them.
The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Sections 1.1 and 1.2). M. fallax is able to survive in the soil for long time and therefore could potentially enter with infested soil/growing media. However, the growing media is certified and heat‐treated by commercial suppliers during production to eliminate pests and diseases (Dossier Sections 1.1 and 1.2).
Uncertainties
-
–
No information is available on the provenance of new plants other than Betula used for plant production in the nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the nematode to enter the nurseries via infected roots of new seedlings of Betula and plants of other species used for plant production in the area. The entry of the nematode with seeds and the growing media the Panel considers as not possible.
A.2.2.3. Possibility of spread within the nursery
B. pendula and B. pubescens plants are either grown in containers (cells, pots, tubes, etc.) outdoors in the open air or in field. Cell‐grown trees may be grown in greenhouses, however most plants will be field grown or field grown in containers (Dossier Sections 1.1 and 1.2). Mother plants of B. pendula are present in one of the nurseries (Dossier Sections 1.1 and 1.2).
The nematode can infect other suitable plants such as Acer palmatum, Cornus sanguinea, Laburnum anagyroides and Lonicera xylosteum, present within the nurseries (Dossier Sections 3.1 and 3.2).
M. fallax can spread within the nurseries by movement of soil, water, infested plant material and contaminated tools, contaminated shoes and machinery. Tools used in the nurseries are disinfected after operation on a stock and before being used on a different plant species (Dossier Sections 1.1 and 1.2); however, no information is available on the measures to reduce the risk of contamination of machinery, shoes or other material (i.e. package, bags, etc.).
Uncertainties
-
–
Possibility that the pest can spread via contaminated soil adhering to shoes, machinery or other material.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the nematode within the nurseries is possible either by movement of infested soil (also via machinery, shoes and other material) water and plant material.
A.2.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database there are no records of notification of Betula plants for planting neither from the UK nor from other countries due to the presence of M. fallax between the years 1995 and January 2024 (EUROPHYT, 2024; TRACES‐NT, 2024).
A.2.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on M. fallax is provided. The description of the risk mitigation measures currently applied in the UK is provided in the Table 8.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
As the plant passport is very similar to the EU one, the plants shall be free from quarantine pests and RNQPs.
Uncertainties
|
| 2 | Physical separation | Yes |
Physical separation from the surroundings and from soil would reduce infections.
Uncertainties
|
| 3 | Certified plant material | Yes |
Seedlings could be a pathway for the nematode.
Uncertainties
|
| 4 | Growing media | Yes |
Heat treatment and protection of the treated growing media is effective against the nematode.
Uncertainties
|
| 5 | Surveillance, monitoring and sampling | Yes |
This measure can have some effect against the nematode.
Uncertainties
|
| 6 | Hygiene measures | Yes |
This measure can have some effect against the nematode.
Uncertainties
|
| 7 | Removal of infested plant material | Yes |
This measure can have some effect against the nematode as the removal of infested plants will reduce the inoculum.
Uncertainties
|
| 8 | Irrigation water | Yes |
Measures aiming at cleaning the irrigation water including filtering can have some effects against nematodes as they can spread via irrigation water.
Uncertainties
|
| 9 | Application of pest control measures | No | Not relevant. No nematicides are used in the nurseries. |
| 10 | Measures against soil pests | Yes |
Separation of the pots from soil is effective against the nematode.
Uncertainties
|
| 11 | Inspections and management of plants before export | Yes |
This assessment can have some effect against the nematode.
Uncertainties
|
| 12 | Separation during transport to the destination | No | Not relevant. The nematode cannot spread between the roots of the plants when transported to the EU. |
A.2.5. Overall likelihood of pest freedom for bare root plants
The scenarios applied in the elicitation for Acer campestre in a previous EFSA opinion (EFSA PLH Panel, 2023) were considered in the current elicitation.
A.2.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare root plants
This scenario assumes that M. fallax has a restricted distribution in the UK, and that the nurseries are under a low pest pressure from the surroundings. In the case of whips, the growing medium is pest‐free. Young plants have had few contacts with soil and have also smaller root systems with a restricted distribution in soil and hence offering fewer opportunities for nematode infection.
A.2.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare root plants
This scenario assumes that M. fallax is more widely distributed in the UK than anticipated, and that the nurseries are under a high pest pressure from the surroundings. The scenario assumes also that symptoms are overlooked during production due to their unspecific nature, and that root galls are not easily detectable at inspection before export. In case of older plants, the production may have involved longer period of soil contact. In addition, older plants have more extended root systems offering more opportunities for nematode infection.
A.2.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare root plants (Median)
The general distribution for Acer is relevant also for Betula. The reduction in the median value reflects that Betula is a less susceptible host for M. fallax compared to Acer.
A.2.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The position of Q1 and Q3 reflect the high uncertainty due to the mix of commodities, and differences in soil exposure times.
A.2.5.5. Elicitation outcomes of the assessment of the pest freedom for Meloidogyne fallax on bare root plants
The following Tables show the elicited and fitted values for pest infestation (Table A.9) and pest freedom (Table A.10).
TABLE A.9.
Elicited and fitted values of the uncertainty distribution of pest infestation by Meloidogyne fallax per 10,000 plants/bundles of bare root plants.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 1 | 28 | 55 | 100 | 200 | ||||||||||
| EKE results | 1.32 | 3.04 | 5.76 | 11.0 | 18.0 | 26.9 | 36.2 | 57.0 | 82.5 | 98.3 | 118 | 139 | 163 | 182 | 200 |
Note: The EKE results is the BetaGeneral (1.1049, 3.0949, 0, 255) distribution fitted with @Risk version 7.6.
Based on the numbers of estimated infested bundles the pest freedom was calculated (i.e. = 10,000 – number of infested plants/bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.10.
TABLE A.10.
The uncertainty distribution of plants free of Meloidogyne fallax per 10,000 plants/bundles of bare root plantscalculated by Table A.9.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited Values | 9800.0 | 9900.0 | 9945.0 | 9972.0 | 9999.0 | ||||||||||
| EKE results | 9800 | 9818 | 9837 | 9861 | 9882 | 9902 | 9918 | 9943 | 9964 | 9973 | 9982 | 9989 | 9994 | 9997 | 9999 |
Note: The EKE results are the fitted values.
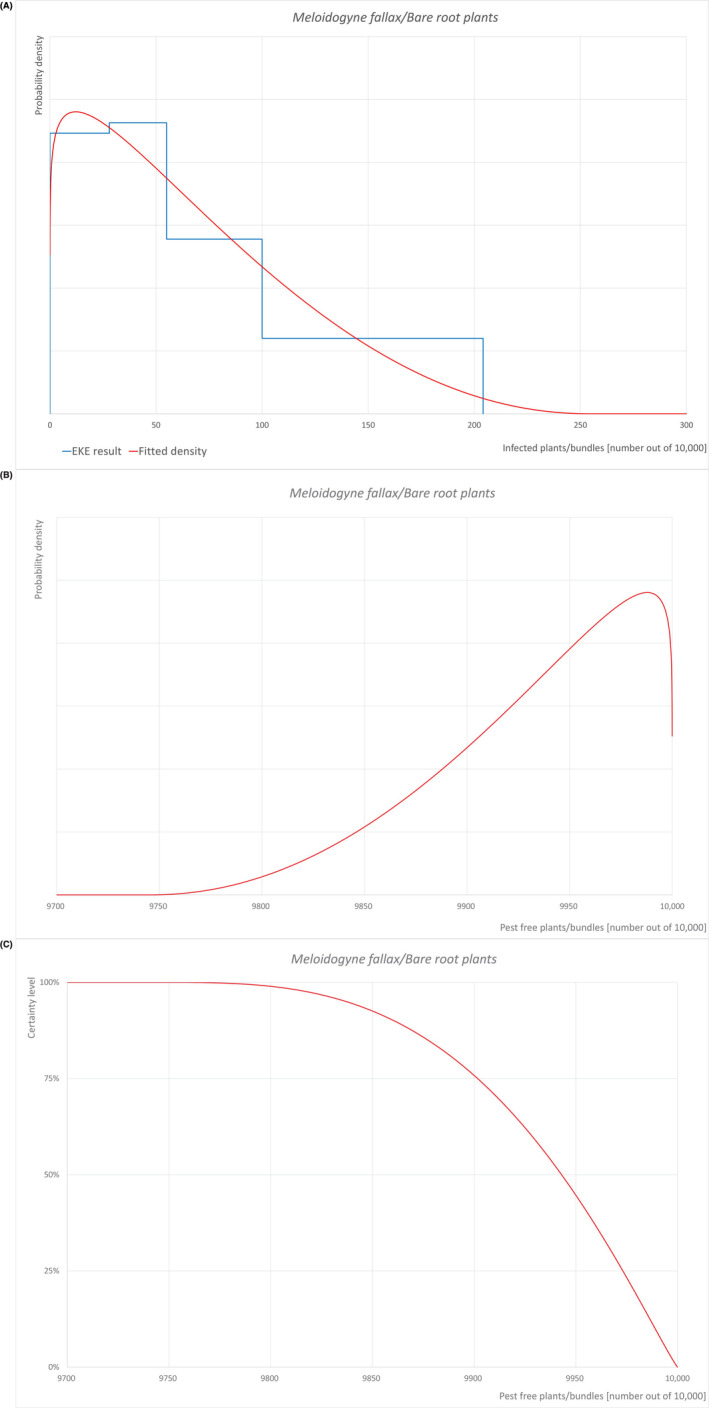
FIGURE A.5 (A) Elicited uncertainty of pest infestation per 10,000 plants/bundles of bare root plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (B) uncertainty of the proportion of pest‐free plants/bundles per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (C) descending uncertainty distribution function of pest infestation per 10,000 plants/bundles.
A.2.6. Overall likelihood of pest freedom for plants in pots
The scenarios applied in the elicitation for Acer campestre in a previous EFSA opinion (EFSA PLH Panel, 2023) were considered in the current elicitation.
A.2.6.1. Reasoning for a scenario which would lead to a reasonably low number of infested plants in pots
This scenario assumes that M. fallax has a restricted distribution in the UK, and that the nurseries are under a low pest pressure from the surroundings. The growing medium used is pest‐free and the plants in pots are grown without soil contact.
A.2.6.2. Reasoning for a scenario which would lead to a reasonably high number of infested plants in pots
This scenario assumes that M. fallax is more widely distributed in the UK than anticipated, and that the nurseries are under a high pest pressure from the surroundings. It also assumes that symptoms are overlooked during production due to their unspecific nature, and that root galls are not easily detectable at inspection before export.
A.2.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested plants in pots (Median)
The general distribution for Acer is relevant also for Betula. The position of the median follows the general distribution of values for Acer with a reduction to lower values. However, the values are kept higher than for bare‐rooted plants of Betula because the lack of root inspection in potted plants.
A.2.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The position of Q1 and Q3 reflects the high uncertainty.
A.2.6.5. Elicitation outcomes of the assessment of the pest freedom for Meloidogyne fallax on plants in pots
The following Tables show the elicited and fitted values for pest infestation (Table A.11) and pest freedom (Table A.12).
TABLE A.11.
Elicited and fitted values of the uncertainty distribution of pest infestation by Meloidogyne fallax per 10,000 plants/bundles.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 1 | 30 | 60 | 115 | 230 | ||||||||||
| EKE | 1.05 | 2.63 | 5.29 | 10.7 | 18.3 | 28.2 | 38.9 | 63.2 | 93.4 | 112 | 136 | 161 | 188 | 210 | 231 |
Note: The EKE results is the BetaGeneral (1.0047, 2.7804, 0, 285) distribution fitted with @Risk version 7.6.
Based on the numbers of estimated infested bundles the pest freedom was calculated (i.e. = 10,000 – number of infested plants/bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.12.
TABLE A.12.
The uncertainty distribution of plants free of Meloidogyne fallax per 10,000 plants/bundles calculated by Table A.11.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9770.0 | 9885.0 | 9940.0 | 9970.0 | 9999.0 | ||||||||||
| EKE results | 9769 | 9790 | 9812 | 9839 | 9864 | 9888 | 9907 | 9937 | 9961 | 9972 | 9982 | 9989 | 9995 | 9997 | 9999 |
Note: The EKE results are the fitted values.
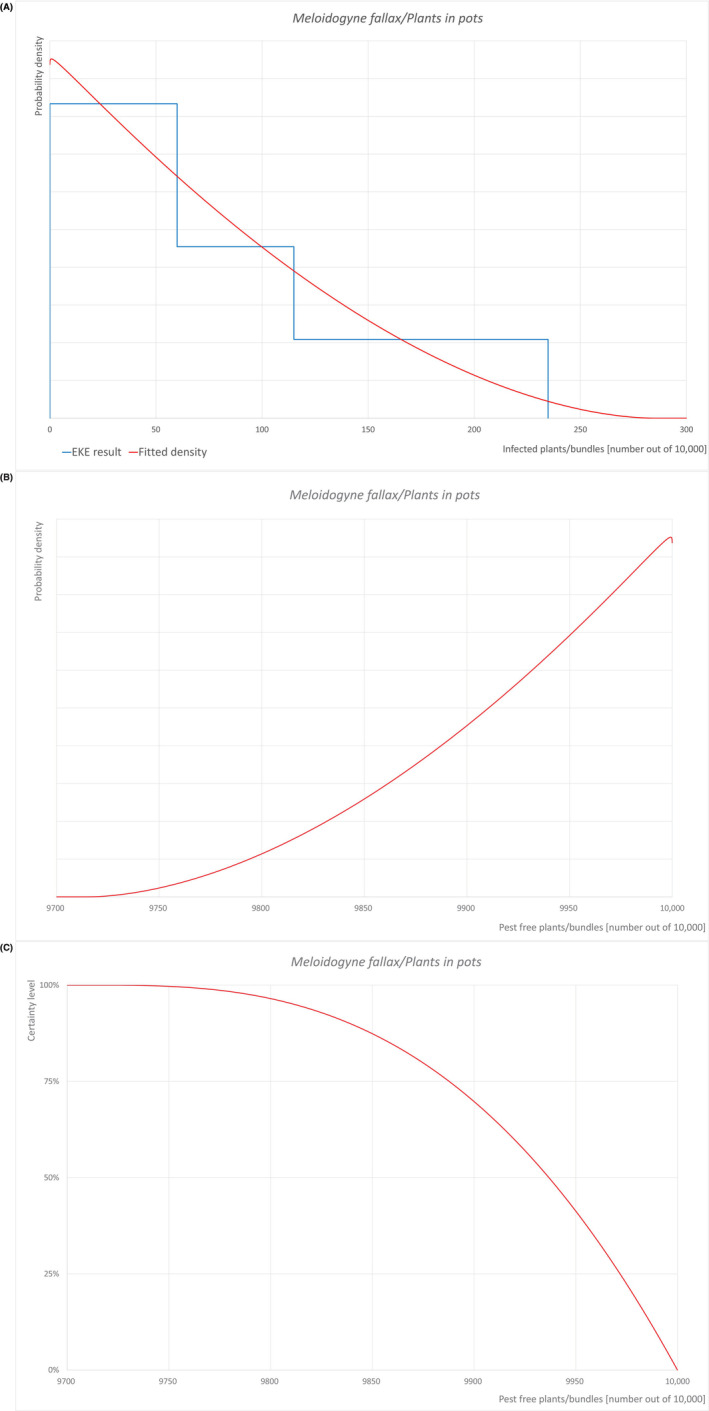
FIGURE A.6 (A) Elicited uncertainty of pest infestation per 10,000 plants/bundles of plants in pots (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (B) uncertainty of the proportion of pest free plants/bundles per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (C) descending uncertainty distribution function of pest infestation per 10,000 plants/bundles.
A.2.7. Overall likelihood of pest freedom for specimen trees
The scenarios applied in the elicitation for Acer campestre in a previous EFSA opinion (EFSA PLH Panel, 2023) were considered in the current elicitation.
A.2.7.1. Reasoning for a scenario which would lead to a reasonably low number of infested specimen trees
This scenario assumes that M. fallax has a restricted distribution in the UK, and that the nurseries are under a low pest pressure from the surroundings. In the case of young trees there has been few contacts with soil. Young trees also have smaller root systems which offer fewer opportunities for nematode infection.
A.2.7.2. Reasoning for a scenario which would lead to a reasonably high number of infested specimen trees
This scenario assumes that M. fallax is more widely distributed in the UK than anticipated. The nurseries are under a high pest pressure from the surroundings. During production symptoms are overlooked due to their unspecific nature. In case of older trees the production may have involved longer period (up to 9 years) of soil contact. Older plants also have more extended root systems which may have offered more points for nematode infection. Washing of large root systems is not effective and symptoms may hide under remaining clumps of soil.
A.2.7.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested specimen trees (Median)
The general distribution for Acer campestre 1–15‐year‐old plants in pots is relevant also for Betula. The position of the median follows the general distribution of values for Acer, but with a reduction to lower values since Betula is a less susceptible host compared to Acer.
A.2.7.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The position of Q1 and Q3 reflects the high uncertainty on the median estimate in both directions. Q3 is set slightly closer to the mean in order to compensate for the slightly high value of the worst‐case scenario.
A.2.7.5. Elicitation outcomes of the assessment of the pest freedom for Meloidogyne fallax on specimen trees
The following Tables show the elicited and fitted values for pest infestation (Table A.13) and pest freedom (Table A.14).
TABLE A.13.
Elicited and fitted values of the uncertainty distribution of pest infection by Meloidogyne fallax per 10,000 plants.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 1 | 51 | 100 | 180 | 300 | ||||||||||
| EKE | 1.72 | 4.39 | 8.92 | 18.3 | 31.2 | 48.1 | 65.8 | 105 | 150 | 176 | 207 | 237 | 265 | 284 | 300 |
Note: The EKE results is the BetaGeneral (0.98296, 1.7313, 0, 323) distribution fitted with @Risk version 7.6.
Based on the numbers of estimated infested bundles the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.14.
TABLE A.14.
The uncertainty distribution of plants free of Meloidogyne fallax per 10,000 plants calculated by Table A.13.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9700 | 9820 | 9900 | 9949 | 9999 | ||||||||||
| EKE results | 9700 | 9716 | 9735 | 9763 | 9793 | 9824 | 9850 | 9895 | 9934 | 9952 | 9969 | 9982 | 9991 | 9996 | 9998 |
Note: The EKE results are the fitted values.
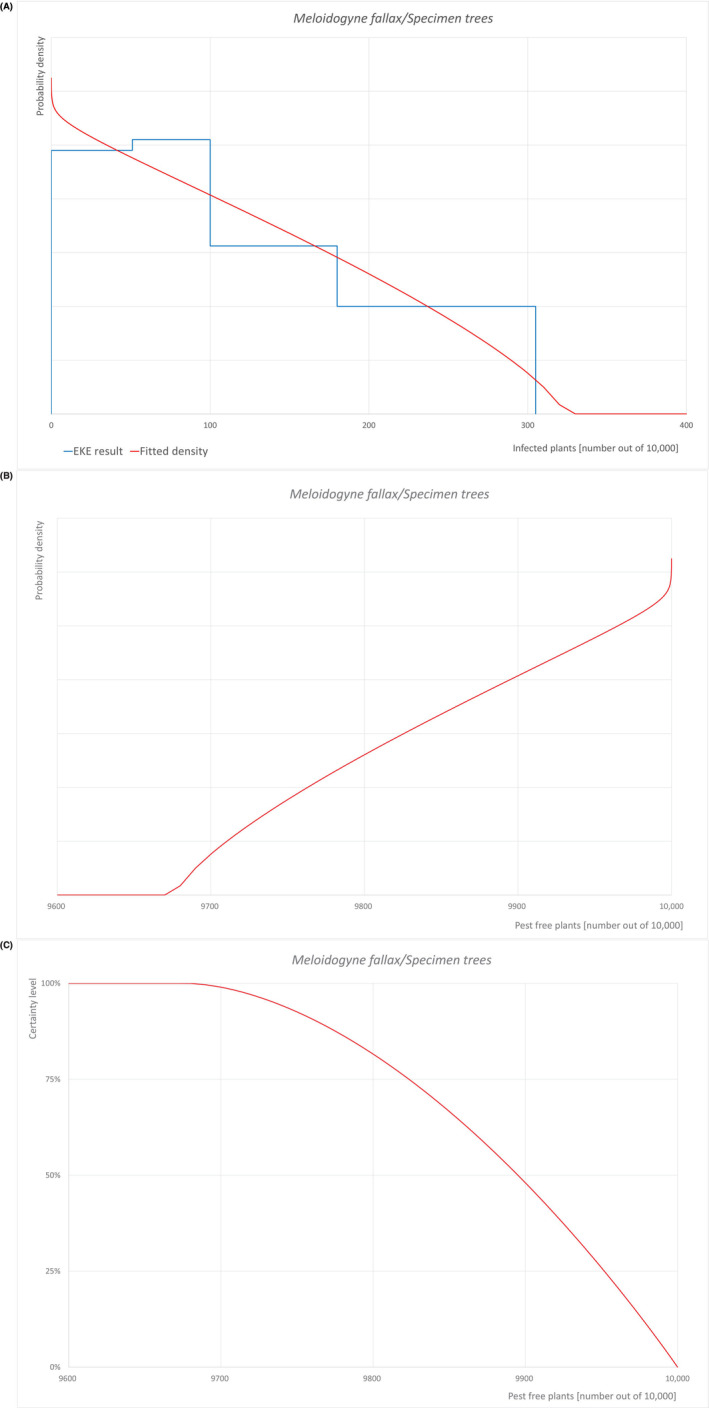
FIGURE A.7 (A) Elicited uncertainty of pest infestation per 10,000 plants of specimen trees (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (B) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (C) descending uncertainty distribution function of pest infestation per 10,000 plants.
A.2.8. Reference list
CABI (Centre for Agriculture and Bioscience International). (2021). Datasheet Meloidogyne fallax (false Columbia root‐knot nematode). https://www.cabi.org/cpc/datasheet/33241
DEFRA (Department for Environment, Food and Rural Affairs). (2024). UK risk register details for Meloidogyne fallax. https://planthealthportal.defra.gov.uk/pests‐and‐diseases/uk‐plant‐health‐risk‐register//viewPestRisks.cfm?cslref=16540
den Nijs, L. J. M. F., Brinkman, H., & van der Sommen, A. T. C. (2004). A Dutch contribution to knowledge on phytosanitary risk and host status of various crops for Meloidogyne chitwoodi Golden et al., 1980 and M. fallax Karssen, 1996: an overview. Nematology, 6, 303–312. https://doi.org/10.1163/1568541042360492
EFSA (European Food Safety Authority), den Nijs, L., Camilleri, M., Diakaki, M., Schenk, M., & Vos, S. (2019). Pest survey card on Meloidogyne chitwoodi and Meloidogyne fallax. EFSA Supporting Publication, EN‐1572. https://doi.org/10.2903/sp.efsa.2019.en‐1572
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard, C., Dehnen‐Schmutz, K., Di Serio, F., Jacques, M.‐A., Jaques Miret, J. A., Justesen, A. F., MacLeod, A., Magnusson, C. S., Milonas, P., Navas‐ Cortes, J. A., Parnell, S., Potting, R., Reignault, P. L., Thulke, H.‐H., Van der Werf, W., Vicent Civera, A., Yuen, J., Zappalà, L., Battisti, A., Mas, H., Rigling, D., Mosbach‐Schulz, O., & Gonthier, P. (2020). Scientific Opinion on the commodity risk assessment of Acer spp. plants from New Zealand. EFSA Journal, 18(5), 6105. https://doi.org/10.2903/j.efsa.2020.6105
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard, C., Baptista, P., Chatzivassiliou, E., Di Serio, F., Jaques Miret, J. A., Justesen, A. F., MacLeod, A., Magnusson, C. S., Milonas, P., Navas‐Cortes, J. A., Parnell, S., Potting, R., Reignault, P. L., Stefani, E., Thulke, H.‐H., Van der Werf, W., Vicent Civera, A., Yuen, J., Zappalà, L., Battisti, A., Mas, H., Rigling, D., Faccoli, M., Gardi, C., Iacopetti, G., Mikulová, A., Mosbach‐Schulz, O., Stergulc, F., Streissl, F., & Gonthier, P. (2023). Scientific Opinion on the commodity risk assessment of Acer campestre plants from the UK. EFSA Journal, 21(7), 8071. https://doi.org/10.2903/j.efsa.2023.8071
EPPO (European and Mediterranean Plant Protection Organization). (2015). Meloidogyne fallax detected in sports turf in United Kingdom. EPPO Reporting Service, 10.
EPPO (European and Mediterranean Plant Protection Organization). (2016). Diagnostics. PM 7/41 (3). Meloidogyne chitwoodi and M. fallax. EPPO Bulletin, 46, 171–189.
EPPO (European and Mediterranean Plant Protection Organization). (2024a). EPPO A2 List of pests recommended for regulation as quarantine pests, version 2023–09. https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list (accessed 2024‐02‐25).
EPPO (European and Mediterranean Plant Protection Organization). (2024b). Meloidogyne fallax (MELGFA), Categorization. https://gd.eppo.int/taxon/MELGFA/categorization (accessed 2024‐02‐25).
EPPO (European and Mediterranean Plant Protection Organization). (2024c). Meloidogyne fallax (MELGFA), Distribution. https://gd.eppo.int/taxon/MELGFA/distribution (accessed 2024‐02‐25).
EPPO (European and Mediterranean Plant Protection Organization). (2024d). Meloidogyne fallax (MELGFA), Reporting. https://gd.eppo.int/taxon/MELGFA/reporting (accessed 2024‐02‐25).
EPPO (European and Mediterranean Plant Protection Organization). (2024e). EPPO Reporting Service no. 05–2024 Num. article: 2024/107. Update on the situation of Meloidogyne chitwoodi and Meloidogyne fallax in the Netherlands. https://gd.eppo.int/reporting/article‐7857 (accessed 2024‐02‐25).
EPPO (European and Mediterranean Plant Protection Organization). (2024f). Meloidogyne fallax (MELGFA), Hosts. https://gd.eppo.int/taxon/MELGFA/hosts (accessed 2024‐02‐25).
EUROPHYT (European Union Notification System for Plant Health Interceptions). (2024). https://food.ec.europa.eu/plants/plant‐health‐and‐biosecurity/europhyt_en (accessed 2024‐02‐09).
Everatt, M., Eyre, D., & Prior, T. (2016). The nematode Meloidogyne fallax in sports turf: symptoms, biosecurity guidance and control. DEFRA Plant Pest Factsheet, (updated 2017), 5 pp.
Ferris, H. (2024). Nemaplex (The Nematode‐Plant Expert Information System). http://nemaplex.ucdavis.edu/ (accessed 2024‐02‐26).
James, K., Back, M., & Prior, T. (2019). A literature review of the root‐knot nematodes (Meloidogyne species) that pose a threat to potato production in GB. Agriculture and Horticulture Development Board, 11, 45 pp.
Kantor, M., Handoo, Z., Kantor, C., & Carta, L. (2022). Top ten most important U.S.‐regulated and emerging plant‐parasitic nematodes. Horticulturae, 8, 208, 1–26. https://doi.org/10.3390/horticulturae8030208
MacLeod, A., Anderson, H., Follak, S., van der Gaag, D. J., Potting, R., Pruvost, O., Smith, J., Steffek, R., Vloutoglou, I., Holt, J., Karadjova, O., Kehlenbeck, H., Labonne, G., Reynaud, P., Viaene, N., Anthoine, G., Holeva, M., Hostachy, B., Ilieva, Z., Karssen, G., Krumov, V., Limon, P., Meffert, J., Niere, B., Petrova, E., Peyre, J., Pfeilstetter, E., Roelofs, W., Rothlisberger, F., Sauvion, N., Schenck, N., Schrader, G., Schroeder, T., Steinmöller, S., Tjou‐Tam‐Sin, L., Ventsislavov, V., Verhoeven, K., & Wesemael, W. (2012). Pest risk assessment for the European Community plant health: a comparative approach with case studies. Cases: Meloidogyne chitwoodi and M. fallax. Supporting Publications, EN‐319. www.efsa.europa.eu/publications
Topalović, O., Moore, J. F., Janssen, T., Bert, W., & Karssen, G. (2017). An early record of Meloidogyne fallax from Ireland. ZooKeys, 643, 33–52. https://doi.org/10.3897/zookeys.643.11266
TRACES‐NT. (2024). TRAde Control and Expert System. https://webgate.ec.europa.eu/tracesnt (accessed 2024‐02‐09).
Van der Sommen, A., den Nijs, L., & Karssen, G. (2005). The root‐knot nematode Meloidogyne fallax on strawberry in the Netherlands. Plant Disease, 89, 526. https://doi.org/10.1094/pd‐89‐0526a
A.3. Phytophthora ramorum (non‐EU isolates)
A.3.1. Organism information
| Taxonomic information |
Current valid scientific name: Phytophthora ramorum Synonyms: – Name used in the EU legislation: Phytophthora ramorum (non‐EU isolates) Werres, De Cock & Man in 't Veld [PHYTRA] Order: Peronosporales Family: Peronosporaceae Common name: Sudden oak death (SOD), ramorum bleeding canker, ramorum blight, ramorum leaf blight, twig and leaf blight Name used in the Dossier: Phytophthora ramorum |
|
| Group | Oomycetes | |
| EPPO code | PHYTRA | |
| Regulated status |
The pathogen is listed in Annex II of Commission Implementing Regulation (EU) 2019/2072 as Phytophthora ramorum (non‐EU isolates) Werres, De Cock & Man in ‘t Veld [PHYTRA]. The EU isolates of P. ramorum are listed as regulated non quarantine pest (RNQP). The pathogen is included in the EPPO A2 list (EPPO, 2024a). P. ramorum is quarantine in Canada, China, Israel, Mexico, Morocco, South Korea and the UK. It is on A1 list of Brazil, Chile, Egypt, Kazakhstan, Switzerland, Türkiye and EAEU (=Eurasian Economic Union: Armenia, Belarus, Kazakhstan, Kyrgyzstan and Russia) (EPPO, 2024b). |
|
| Pest status in the UK |
P. ramorum is present in the UK (Brown & Brasier, 2007; Dossier Section 2.0; CABI, 2020; EPPO, 2024c). According to the Dossier Section 2.0, European isolates of P. ramorum are present in the UK: not widely distributed and under official control. It has been found in most regions of the UK, but it is more often reported in wetter, western regions. |
|
| Pest status in the EU | P. ramorum is present in the EU and it is currently reported in the following EU Member States: Belgium, Croatia, Denmark, Finland (transient), France, Germany, Ireland, Luxembourg, the Netherlands, Poland, Portugal and Slovenia (EPPO, 2024c). | |
| Host status on Betula pendula and B. pubescens |
P. ramorum was reported to infect Betula pendula in the UK (King et al., 2015; Webber et al., 2010) and Finland (Lilja et al., 2007), although Koch's postulate has not yet been completely fulfilled for this pathosystem (APHIS USDA, 2022). The susceptibility of B. pendula to P. ramorum was assessed as low based on experimental leaf and bark inoculations tests (Sansford et al. 2009). There is no information on other Betula species (including B. pubescens) being hosts. |
|
| PRA information | Pest Risk Assessments available:
|
|
| Other relevant information for the assessment | ||
| Biology |
P. ramorum is most probably native to East Asia (Jung et al., 2021; Poimala & Lilja, 2013). The pathogen is present in Asia (Japan, Vietnam), Europe (Belgium, Croatia, Denmark, Finland, France, Germany, Guernsey, Ireland, Luxembourg, the Netherlands, Norway, Poland, Portugal, Slovenia, the UK), North America (Canada, the US) and South America (Argentina) (EPPO, 2024c). So far there are 12 known lineages of P. ramorum: NA1 and NA2 from North American, EU1 from Europe (including the UK) and North America (Grünwald et al., 2009), EU2 from Northern Ireland and western Scotland (Van Poucke et al., 2012), IC1 to IC5 from Vietnam and NP1 to NP3 from Japan (Jung et al., 2021). P. ramorum is heterothallic oomycete species belonging to clade 8c (Blair et al., 2008) with two mating types: A1 and A2 (Boutet et al., 2010). Phytophthora species generally reproduce through a) dormant (resting) spores which can be either sexual (oospores) or asexual (chlamydospores); and (b) fruiting structures (sporangia) which contain zoospores (Erwin & Ribeiro, 1996). P. ramorum produces sporangia on the surfaces of infected leaves and twigs of host plants. These sporangia can be splash‐dispersed to other close or carried by wind and rain to longer distances. The sporangia germinate to produce zoospores that penetrate and initiate an infection on new hosts. In infected plant material the chlamydospores are produced and can serve as resting structures (Davidson et al., 2005; Grünwald et al., 2008). The pathogen is also able to survive in soil (Shishkoff, 2007). In the west of Scotland, it persisted in soil for at least 2 years after its hosts were removed (Elliot et al., 2013). Oospores were only observed in pairing tests under controlled laboratory conditions (Brasier & Kirk, 2004). Optimal temperatures under laboratory conditions were 16–26°C for growth, 14–26°C for chlamydospore production and 16–22°C for sporangia production (Englander et al., 2006). P. ramorum is mainly a foliar pathogen, however it was also reported to infect shoots, stems and occasionally roots of various host plants (Grünwald et al., 2008, Parke & Lewis, 2007). According to Brown and Brasier (2007), P. ramorum commonly occupies xylem beneath phloem lesions and may spread within xylem and possibly recolonize the phloem from the xylem. P. ramorum can remain viable within xylem for two or more years after the overlying phloem had been excised. P. ramorum can disperse by aerial dissemination, water, movement of infested plant material and soil containing propagules on footwear, tires of trucks and mountain bikes, or the feet of animals (Brasier, 2008; Davidson et al., 2002). Infected foliar hosts can be a major source of inoculum, which can lead to secondary infections on nearby host plants. Important foliar hosts in Europe are Rhododendron spp. and Larix kaempferi (Brasier & Webber, 2010, Grünwald et al., 2008). Possible pathways of entry for P. ramorum are plants for planting (excluding seed and fruit) of known susceptible hosts; plants for planting (excluding seed and fruit) of non‐host plant species accompanied by contaminated attached growing media; soil/growing medium (with organic matter) as a commodity; soil as a contaminant; foliage or cut branches; seed and fruits; susceptible (isolated) bark and susceptible wood (EFSA PLH Panel, 2011). P. ramorum caused rapid decline of Lithocarpus densiflorus and Quercus agrifolia in forests of California and Oregon (Rizzo et al., 2005) and Larix kaempferi in plantations of southwest England (Brasier & Webber, 2010). |
|
| Symptoms | Main type of symptoms |
P. ramorum causes different types of symptoms depending on the host species and the plant tissue infected. According to DEFRA (2008) P. ramorum causes three different types of disease:
The only reported symptoms on Betula pendula were necrotic lesions on leaves in Finland (Lilja et al., 2007) and ramorum canker in the UK (DEFRA, 2015). |
| Presence of asymptomatic plants |
If roots are infected by P. ramorum, the plants can be without above‐ground symptoms for months until developmental or environmental factors trigger disease expression (Roubtsova & Bostock, 2009; Thompson et al., 2021). Application of some fungicides may reduce symptoms and therefore mask infection, making it more difficult to determine whether the plant is pathogen‐free (DEFRA, 2008). |
|
| Confusion with other pests |
Various symptoms caused by P. ramorum can be confused with other pathogens, such as: canker and foliar symptoms caused by other Phytophthora species (P. cinnamomi, P. citricola and P. cactorum); leaf lesions caused by rust in early stages; leafspots caused by sunburn; dieback of twigs and leaves caused by Botryosphaeria dothidea (Davidson et al., 2003). P. ramorum can be easily distinguished from other pathogens, including Phytophthora species based on morphology (Grünwald et al., 2008) and molecular tests. |
|
| Host plant range |
P. ramorum has a very wide host range, which is expanding. Main host plants include Camellia spp., Larix decidua, L. kaempferi, Pieris spp., Rhododendron spp., Syringa vulgaris, Viburnum spp. and the North American trees species, Lithocarpus densiflorus and Quercus agrifolia (EPPO 2024d). Further proven hosts confirmed by Koch's postulates are Abies grandis, A. magnifica, Acer circinatum, A. macrophyllum, A. pseudoplatanus, Adiantum aleuticum, A. jordanii, Aesculus californica, A. hippocastanum, Arbutus menziesii, A. unedo, Arctostaphylos columbiana, A. glauca, A. hooveri, A. manzanita, A. montereyensis, A. morroensis, A. pilosula, A. pumila, A. silvicola, A. viridissima, Betula pendula, Calluna vulgaris, Castanea sativa, Ceanothus thyrsiflorus, Chamaecyparis lawsoniana, Chrysolepis chrysophylla, Cinnamomum camphora, Corylus cornuta, Fagus sylvatica, Frangula californica, Frangula purshiana, Fraxinus excelsior, Gaultheria procumbens, G. shallon, Griselinia littoralis, Hamamelis virginiana, Heteromeles arbutifolia, Kalmia spp., Larix × eurolepis, Laurus nobilis,, Lonicera hispidula, Lophostemon confertus, Loropetalum chinense, Magnolia × loebneri, M. oltsopa, M. stellata, Mahonia aquifolium, Maianthemum racemosum, Parrotia persica, Photinia fraseri, Phoradendron serotinum subsp. macrophyllum, Photinia × fraseri, Prunus laurocerasus, Pseudotsuga menziesii var. menziesii, Quercus cerris, Q. chrysolepis, Q. falcata Q. ilex, Q. kelloggii, Q. parvula var. shrevei, Q. petraea, Q. robur, Rosa gymnocarpa, Salix caprea, Sequoia sempervirens, Taxus baccata, Trientalis latifolia, Umbellularia californica, Vaccinium myrtillus, V. ovatum, V. parvifolium and Vinca minor (APHIS USDA, 2022; Cave et al., 2008; Farr & Rossman, 2024; EPPO, 2024d). |
|
| Reported evidence of impact | P. ramorum is an EU quarantine pest. | |
| Evidence that the commodity is a pathway | P. ramorum was continuously intercepted in the EU on different plant species intended for planting (EUROPHYT, 2024; TRACES‐NT, 2024) and according to EFSA PLH Panel (2011), P. ramorum can travel with plants for planting. Therefore, plants for planting are a possible pathway of entry for P. ramorum. | |
| Surveillance information |
P. ramorum: at growing sites: infested plants are destroyed and potentially infested plants are ‘held’ (prohibited from moving). The UK has a containment policy in the wider environment with official action taken to remove infected trees (Dossier Sections 1.1 and 1.2). As part of an annual survey at ornamental retail and production sites (frequency of visits determined by a decision matrix), P. ramorum is inspected for on common hosts plants. An additional inspection, during the growing period, is carried out at plant passport production sites. Inspections are carried out at a survey to 300 non‐woodland wider environment sites annually (Dossier Sections 1.1 and 1.2). |
|
A.3.2. Possibility of pest presence in the nursery
A.3.2.1. Possibility of entry from the surrounding environment
P. ramorum is present in the UK, it has been found in most regions of the UK, but it is more often reported in wetter, western regions (Dossier Section 2.0).
The possible entry of P. ramorum from surrounding environment to the nurseries may occur through aerial dissemination, water, animals, machinery and footwear (Brasier, 2008; Davidson et al., 2002).
P. ramorum has wide host range and can infect number of different plants. Suitable plants like Acer pseudoplatanus, Camellia spp., Chamaecyparis lawsoniana, Fraxinus spp., Larix kaempferi, Larix spp., Quercus spp., Quercus petraea, Q. robur, Pieris spp., Prunus laurocerasus, Rhododendron spp., Taxus baccata and Viburnum spp. are present in hedges and woodland in the surrounding areas of nurseries (Dossier Sections 1.1, 1.2 and 5.1).
Uncertainties
-
–
The dispersal range of P. ramorum sporangia.
-
–
No information available on the distance of the nurseries to sources of pathogen in the surrounding environment.
-
–
No information is provided whether machinery from outside the nursery is used inside the nursery.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nurseries from surrounding environment. In the surrounding area, suitable hosts are present and the pathogen can spread by wind, rain and infested soil propagules on feet of animals entering the nurseries.
A.3.2.2. Possibility of entry with new plants/seeds
The starting materials are either seeds or seedlings and shoots/buds when grafted plants are produced. Seeds are certified and come from the UK. Seedlings are also certified and are either from the UK or the EU (the Netherlands) (Dossier Sections 1.1 and 1.2).
In addition to B. pendula and B. pubescens plants, the nurseries also produce other plants (Dossier Sections 3.1, 3.2 and 5.1). These include many suitable hosts for the pathogen (such as Abies spp., Acer spp., Aesculus spp., Arbutus spp., Calluna spp., Castanea spp., Fagus spp., Larix kaempferi, Larix spp., Quercus spp., Prunus spp., Rhododendron spp., Viburnum spp., etc.). However, there is no information on how and where the plants are produced. Therefore, if the plants are first produced in another nursery, the pathogen could possibly travel with them.
The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Sections 1.1 and 1.2). P. ramorum is able to survive in soil (Shishkoff, 2007) and therefore could potentially enter with infested soil/growing media. However, the growing media is certified and heat‐treated by commercial suppliers during production to eliminate pests and diseases (Dossier Sections 1.1 and 1.2).
Uncertainties
-
–
No information is available on the provenance of plants other than Betula used for plant production in the area of the nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nurseries with new seedlings of Betula and new plants of other species used for plant production in the area. The entry of the pathogen with seeds and the growing media the Panel considers as not possible.
A.3.2.3. Possibility of spread within the nursery
Betula plants are either grown in containers (cells, pots, tubes, etc.) or in field. Cell‐grown trees may be grown in greenhouses, however most plants will be field grown or field grown in containers (Dossier Sections 1.1 and 1.2). One of the nurseries have mother plants of B. pendula (Dossier Sections 1.1 and 1.2), which could serve as a reservoir of the pathogen.
The pathogen can infect other suitable plants (such as Abies spp., Aesculus spp., Castanea spp., Larix spp., Fagus spp., Quercus spp., Rhododendron spp., etc.) present within the nurseries and hedges surrounding the nurseries (Prunus spp., Taxus baccata) (Dossier Sections 1.1, 1.2, 3.1, 3.2 and 5.1).
Phytophthora ramorum can spread within the nurseries by aerial dissemination, soil, water, movement of infested plant material, machinery, footwear and animals (Brasier, 2008; Davidson et al., 2002).
Uncertainties
-
–
None.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pathogen within the nurseries is possible either by aerial dissemination, animals, movement of infested plant material, soil and water.
A.3.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database there are no records of notification of Betula plants for planting neither from the UK nor from other countries due to the presence of P. ramorum between the years 1995 and January 2024 (EUROPHYT, 2024; TRACES‐NT, 2024).
A.3.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on P. ramorum is provided. The description of the risk mitigation measures currently applied in the UK is provided in the Table 8.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
The registration and the release of the UK plant passport should be enough to warrant pest‐free plant material for a quarantine pest in the UK. P. ramorum is a quarantine organism in the UK and targeted by this measure.
Uncertainties
|
| 2 | Physical separation | No | Not relevant |
| 3 | Certified plant material | Yes |
P. ramorum is a quarantine organism in the UK and targeted by this measure.
Uncertainties
|
| 4 | Growing media | Yes |
This measure should ensure pest‐free growing media and is expected to prevent the introduction of the pathogen into the nurseries with growing media.
Uncertainties
|
| 5 | Surveillance, monitoring and sampling | Yes |
This measure has an effect as the pathogen would be detected on nursery‐grown plants, as well as on incoming plant material and growing media, and suspected plant material quarantined.
Uncertainties
|
| 6 | Hygiene measures | Yes |
General hygiene measures will reduce the likelihood of the pathogen being spread by tools and equipment, although this is not a major pathway for the pest.
Uncertainties
|
| 7 | Removal of infested plant material | Yes |
This measure could have some effect by removing potentially infested plant material, thus reducing the spread of the pathogen within the nursery.
Uncertainties
|
| 8 | Irrigation water | Yes |
Testing of irrigation water would detect the pathogen, which can spread by water. Overhead irrigation could favour foliar infections and spread of the pathogen by water splash.
Uncertainties
|
| 9 | Application of pest control measures | Yes |
Some fungicides could reduce the likelihood of foliar infection by the pathogen.
Uncertainties
|
| 10 | Measures against soil pests | Yes |
This measure could have some effect by preventing root contact with soil where the pathogen may be present.
Uncertainties
|
| 11 | Inspections and management of plants before export | Yes |
P. ramorum is a quarantine organism in the UK and the EU and this measure is expected to reduce the likelihood of infested plants being exported.
Uncertainties
|
| 12 | Separation during transport to the destination | No | Not relevant |
A.3.5. Overall likelihood of pest freedom for graftwood/budwood
A.3.5.1. Reasoning for a scenario which would lead to a reasonably low number of infected graftwood/budwood
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. The plants are exposed to the pathogen for only short period of time. The scenario assumes Betula spp. to be minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.3.5.2. Reasoning for a scenario which would lead to a reasonably high number of infected graftwood/budwood
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. The scenario assumes that the pathogen causes bark infections on the commodity. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.3.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected graftwood/budwood (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings, and a limited susceptibility of Betula spp. The pathogen is a regulated quarantine pest in the UK and under official control.
A.3.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on the occurrence of the pathogen in the nurseries and the surroundings and the susceptibility of Betula spp. results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.3.5.5. Elicitation outcomes of the assessment of the pest freedom for Phytophthora ramorum on graftwood/budwood
The following Tables show the elicited and fitted values for pest infection (Table A.15) and pest freedom (Table A.16).
TABLE A.15.
Elicited and fitted values of the uncertainty distribution of pest infection by Phytophthora ramorum per 10,000 bundles.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 6 | 12 | 22 | 45 | ||||||||||
| EKE | 0.270 | 0.631 | 1.21 | 2.33 | 3.84 | 5.78 | 7.82 | 12.4 | 18.1 | 21.6 | 26.1 | 30.9 | 36.3 | 40.6 | 45.0 |
Note: The EKE results is the BetaGeneral (1.0863, 3.2055, 0, 58.3) distribution fitted with @Risk version 7.6.
Based on the numbers of estimated infected bundles the pest freedom was calculated (i.e. = 10,000 – number of infected bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.16.
TABLE A.16.
The uncertainty distribution of plants free of Phytophthora ramorum per 10,000 bundles calculated by Table A.15.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9955 | 9978 | 9988 | 9994 | 10,000 | ||||||||||
| EKE results | 9955 | 9959 | 9964 | 9969 | 9974 | 9978 | 9982 | 9988 | 9992 | 9994 | 9996 | 9997.7 | 9998.8 | 9999.4 | 9999.7 |
Note: The EKE results are the fitted values.
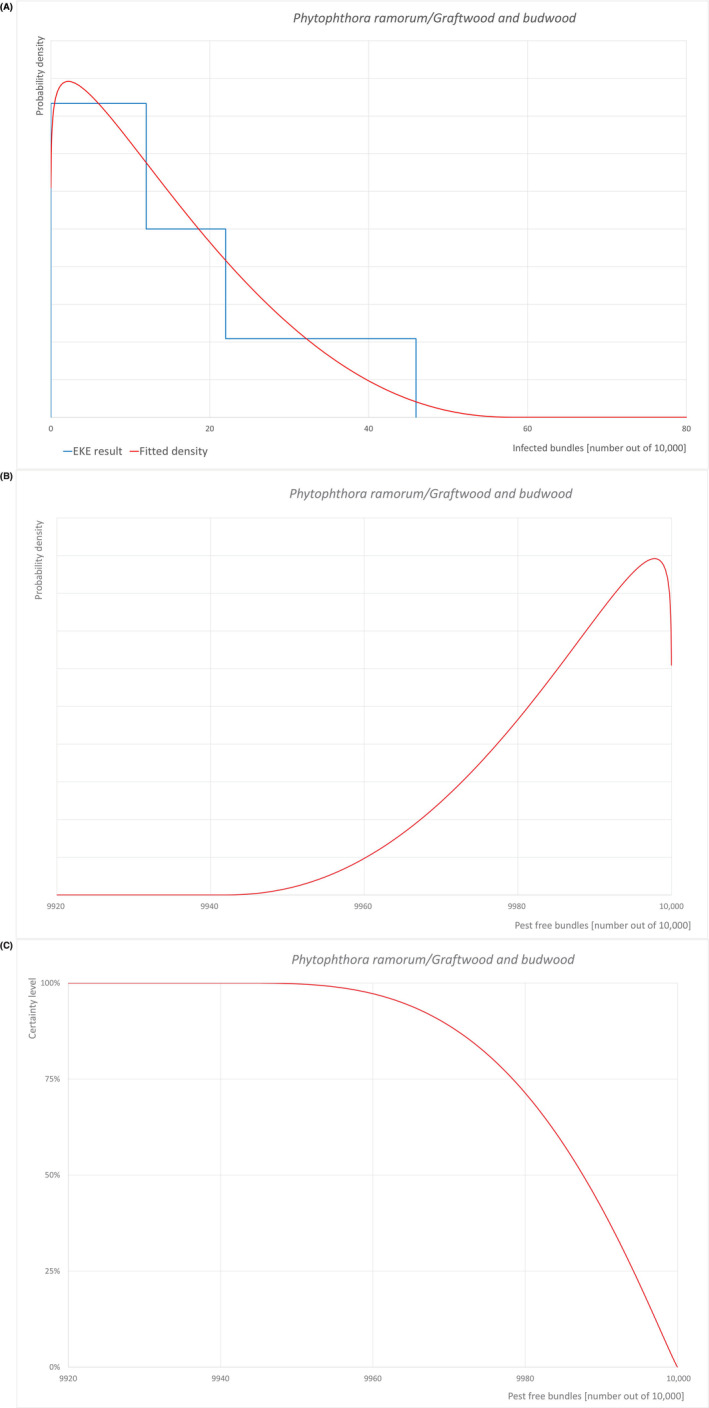
FIGURE A.8 (A) Elicited uncertainty of pest infection per 10,000 bundles of graftwood/budwood (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (B) uncertainty of the proportion of pest‐free bundles per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (C) descending uncertainty distribution function of pest infection per 10,000 bundles.
A.3.6. Overall likelihood of pest freedom for bare root plants
A.3.6.1. Reasoning for a scenario which would lead to a reasonably low number of infected bare root plants
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. The plants are exposed to the pathogen for only short period of time and are exported without leaves. The scenario assumes Betula spp. to be minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.3.6.2. Reasoning for a scenario which would lead to a reasonably high number of infected bare root plants
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. The scenario assumes that the pathogen infects bark and occasionally leaves, which may still be present on the plants at the time of export. Older trees are more likely to become infected due to longer exposure time and larger size. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.3.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bare root plants (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings, and a limited susceptibility of Betula spp. The pathogen is a regulated quarantine pest in the UK and under official control.
A.3.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on the occurrence of the pathogen in the nurseries and the surroundings and the susceptibility of Betula spp. results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.3.6.5. Elicitation outcomes of the assessment of the pest freedom for Phytophthora ramorum on bare root plants
The following Tables show the elicited and fitted values for pest infection (Table A.17) and pest freedom (Table A.18).
TABLE A.17.
Elicited and fitted values of the uncertainty distribution of pest infection by Phytophthora ramorum per 10,000 plants/bundles.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 11 | 20 | 40 | 80 | ||||||||||
| EKE | 0.404 | 0.984 | 1.94 | 3.86 | 6.49 | 9.92 | 13.6 | 21.8 | 32.1 | 38.5 | 46.6 | 55.3 | 64.8 | 72.4 | 80.0 |
Note: The EKE results is the BetaGeneral (1.0357, 2.9697, 0, 101) distribution fitted with @Risk version 7.6.
Based on the numbers of estimated infected bundles the pest freedom was calculated (i.e. = 10,000 – number of infected plants/bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.18.
TABLE A.18.
The uncertainty distribution of plants free of Phytophthora ramorum per 10,000 plants/bundles calculated by Table A.17.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9920 | 9960 | 9980 | 9989 | 10,000 | ||||||||||
| EKE results | 9920 | 9928 | 9935 | 9945 | 9953 | 9961 | 9968 | 9978 | 9986 | 9990 | 9994 | 9996 | 9998 | 9999.0 | 9999.6 |
Note: The EKE results are the fitted values.
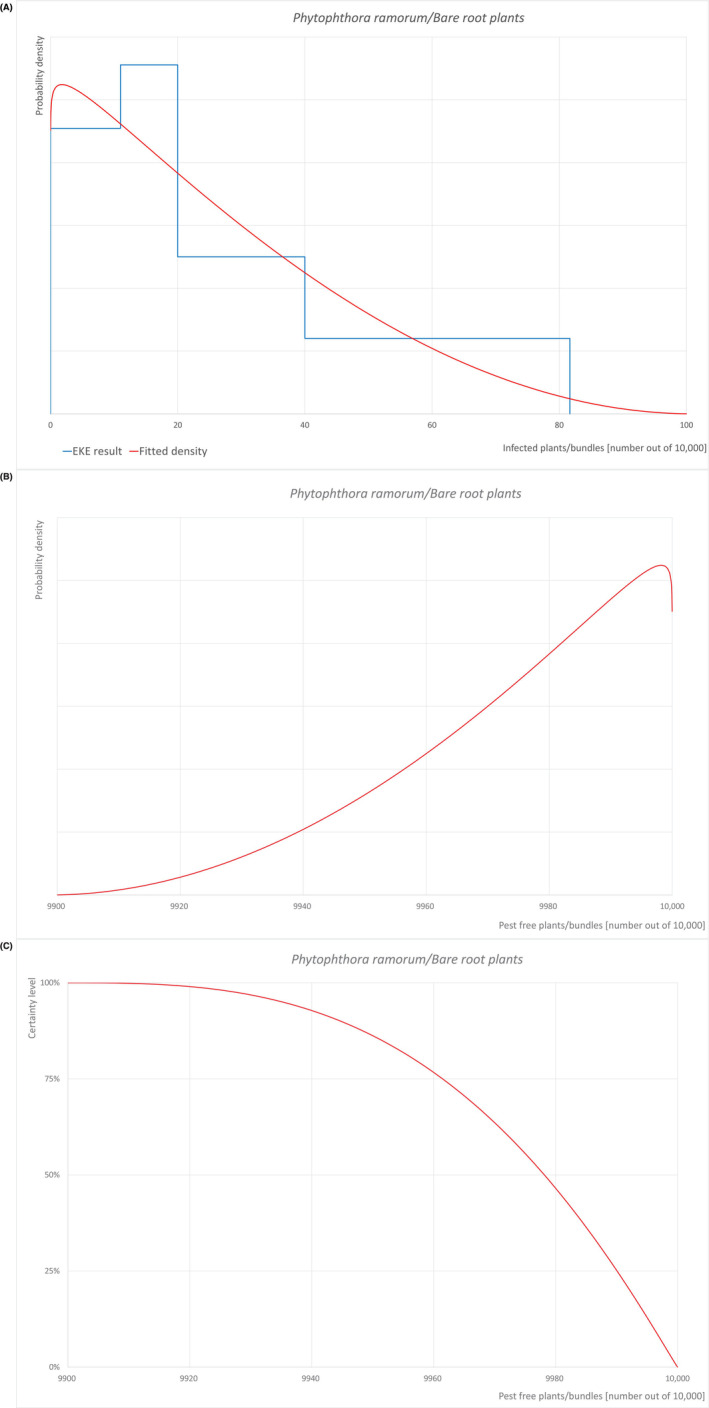
FIGURE A.9 (A) Elicited uncertainty of pest infection per 10,000 plants/bundles of bare root plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (B) uncertainty of the proportion of pest‐free plants/bundles per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (C) descending uncertainty distribution function of pest infection per 10,000 plants/bundles.
A.3.7. Overall likelihood of pest freedom for plants in pots
A.3.7.1. Reasoning for a scenario which would lead to a reasonably low number of infected plants in pots
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Younger plants are exposed to the pathogen for only short period of time and are exported without leaves. The scenario assumes Betula spp. to be minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.3.7.2. Reasoning for a scenario which would lead to a reasonably high number of infected plants in pots
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. The scenario assumes that the pathogen infects bark and occasionally leaves, which may still be present on the plants at the time of export. Older trees are more likely to become infected due to longer exposure time and larger size. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.3.7.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected plants in pots (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings, and a limited susceptibility of Betula spp. The pathogen is a regulated quarantine pest in the UK and under official control.
A.3.7.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on the occurrence of the pathogen in the nurseries and the surroundings and the susceptibility of Betula spp. results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.3.7.5. Elicitation outcomes of the assessment of the pest freedom for Phytophthora ramorum on plants in pots
The following Tables show the elicited and fitted values for pest infection (Table A.19) and pest freedom (Table A.20).
TABLE A.19.
Elicited and fitted values of the uncertainty distribution of pest infection by Phytophthora ramorum per 10,000 plants/bundles.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 11 | 20 | 40 | 80 | ||||||||||
| EKE | 0.404 | 0.984 | 1.94 | 3.86 | 6.49 | 9.92 | 13.6 | 21.8 | 32.1 | 38.5 | 46.6 | 55.3 | 64.8 | 72.4 | 80.0 |
Note: The EKE results is the BetaGeneral (1.0357, 2.9697, 0, 101) distribution fitted with @Risk version 7.6.
Based on the numbers of estimated infected bundles the pest freedom was calculated (i.e. = 10,000 – number of infected plants/bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.20.
TABLE A.20.
The uncertainty distribution of plants free of Phytophthora ramorum per 10,000 plants/bundles calculated by Table A.19.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9920 | 9960 | 9980 | 9989 | 10,000 | ||||||||||
| EKE results | 9920 | 9928 | 9935 | 9945 | 9953 | 9961 | 9968 | 9978 | 9986 | 9990 | 9994 | 9996 | 9998 | 9999.0 | 9999.6 |
Note: The EKE results are the fitted values.
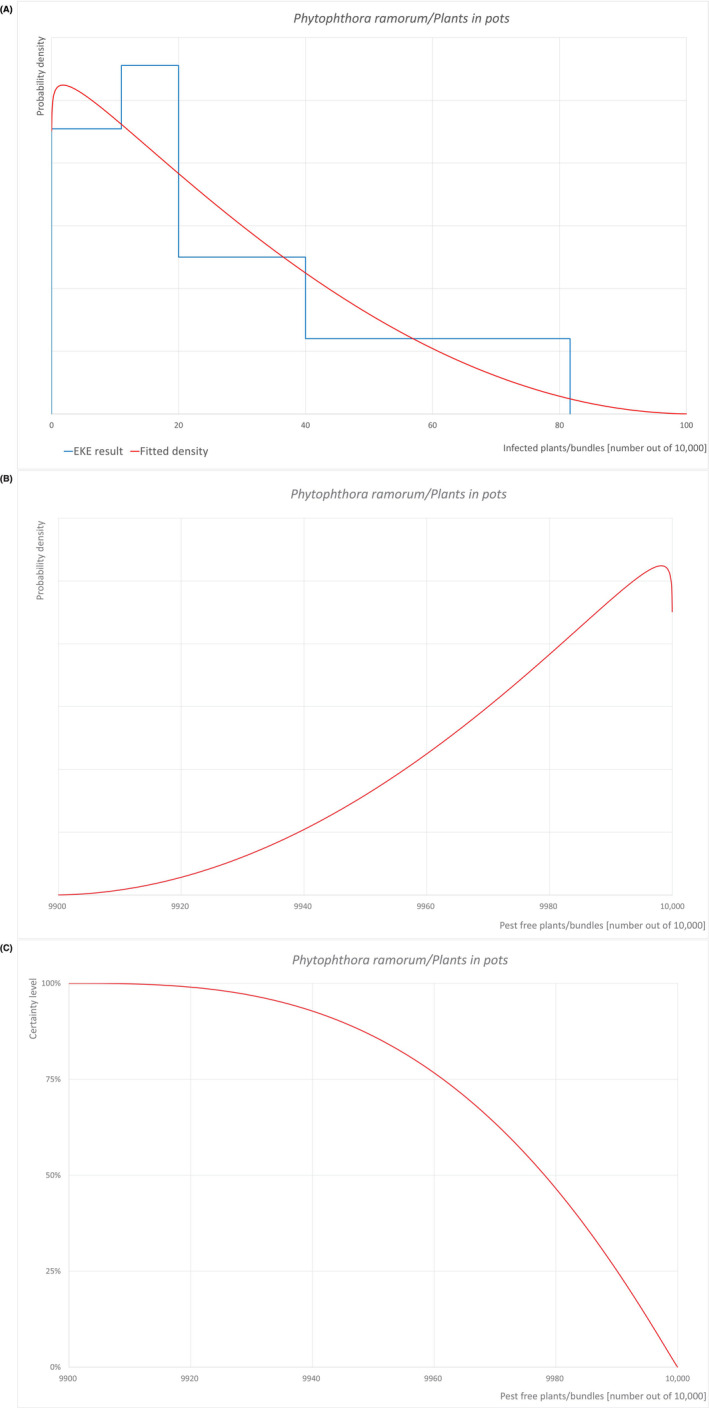
FIGURE A.10 (A) Elicited uncertainty of pest infection per 10,000 plants/bundles of plants in pots (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (B) uncertainty of the proportion of pest‐free plants/bundles per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (C) descending uncertainty distribution function of pest infection per 10,000 plants/bundles.
A.3.8. Overall likelihood of pest freedom for specimen trees
A.3.8.1. Reasoning for a scenario which would lead to a reasonably low number of infected specimen trees
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Plants are exported without leaves. The scenario assumes Betula spp. to be minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.3.8.2. Reasoning for a scenario which would lead to a reasonably high number of infected specimen trees
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. The scenario assumes that the pathogen infects bark and occasionally leaves, which may still be present on the plants at the time of export. Older trees are more likely to become infected due to longer exposure time and larger size. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.3.8.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected specimen trees (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings, and a limited susceptibility of Betula spp. Most of the trees will be younger than 15 years at the time of export. The pathogen is a regulated quarantine pest in the UK and under official control.
A.3.8.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on the occurrence of the pathogen in the nurseries and the surroundings and the susceptibility of Betula spp. results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.3.8.5. Elicitation outcomes of the assessment of the pest freedom for Phytophthora ramorum on specimen trees
The following Tables show the elicited and fitted values for pest infection (Table A.21) and pest freedom (Table A.22).
TABLE A.21.
Elicited and fitted values of the uncertainty distribution of pest infection by Phytophthora ramorum per 10,000 plants.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 15 | 30 | 55 | 100 | ||||||||||
| EKE | 0.535 | 1.34 | 2.69 | 5.46 | 9.29 | 14.3 | 19.5 | 31.3 | 45.5 | 53.9 | 64.1 | 74.5 | 85.2 | 92.9 | 99.9 |
Note: The EKE results is the BetaGeneral (1.0021, 2.1405, 0, 113) distribution fitted with @Risk version 7.6.
Based on the numbers of estimated infected bundles the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.22.
TABLE A.22.
The uncertainty distribution of plants free of Phytophthora ramorum per 10,000 plants calculated by Table A.21.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9900 | 9945 | 9970 | 9985 | 10,000 | ||||||||||
| EKE results | 9900 | 9907 | 9915 | 9925 | 9936 | 9946 | 9955 | 9969 | 9980 | 9986 | 9991 | 9995 | 9997 | 9998.7 | 9999.5 |
Note: The EKE results are the fitted values.
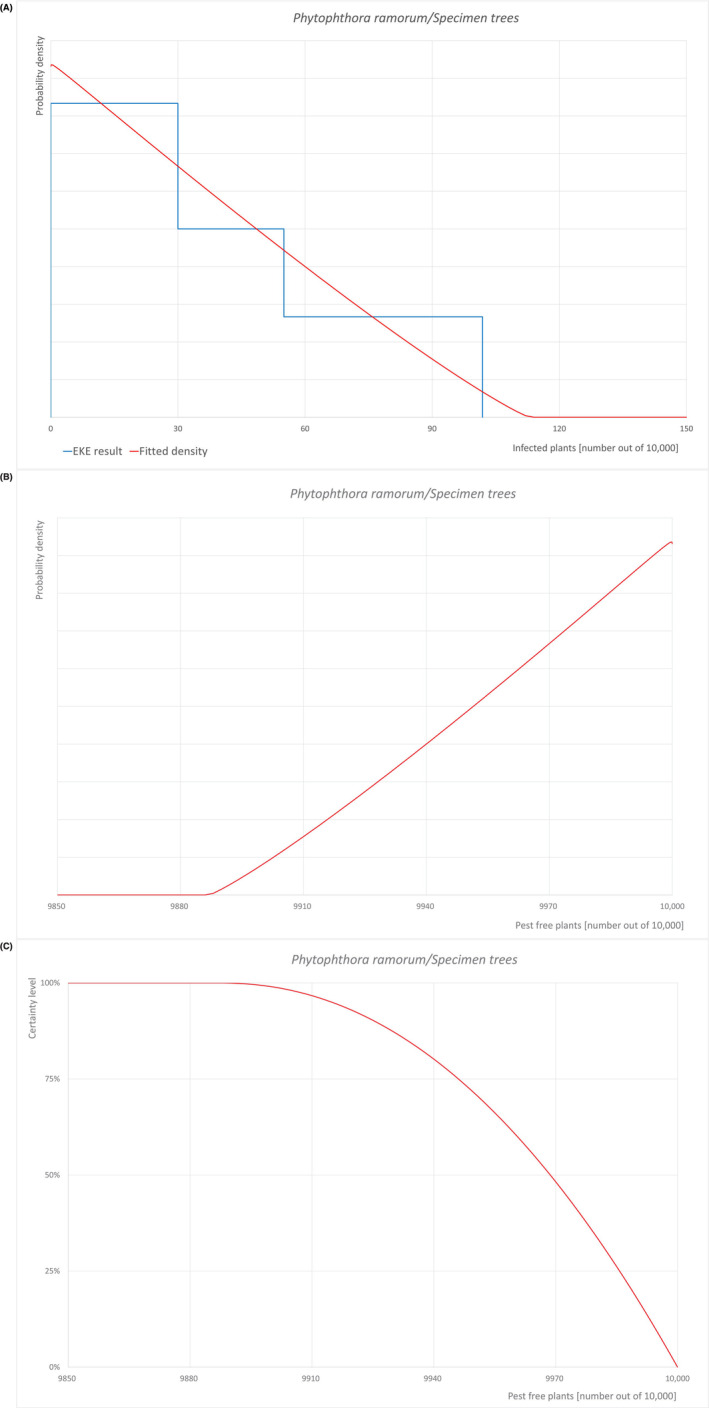
FIGURE A.11 (A) Elicited uncertainty of pest infection per 10,000 plants of specimen trees (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (B) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (C) descending uncertainty distribution function of pest infection per 10,000 plants.
A.3.9. Reference list
APHIS USDA (Animal and Plant Health Inspection Service U.S. Department of Agriculture). (2022). APHIS Lists of Proven Hosts of and Plants Associated with Phytophthora ramorum. September 2022. 12 pp. https://www.aphis.usda.gov/plant_health/plant_pest_info/pram/downloads/pdf_files/usdaprlist.pdf
Blair, J. E., Coffey, M. D., Park, S. Y., Geiser, D. M., & Kang, S. (2008). A multi‐locus phylogeny for Phytophthora utilizing markers derived from complete genome sequences. Fungal Genetics and Biology, 45(3), 266–277. https://doi.org/10.1016/j.fgb.2007.10.010
Boutet, X., Vercauteren, A., Heungens, C., & Kurt, A. (2010). Mating of Phytophthora ramorum: functionality and consequences. In S. J. Frankel, J. T. Kliejunas, and K. M. Palmieri (Eds.), Proceedings of the Sudden Oak Death Fourth Science Symposium (229, pp. 97–100). Albany, CA: US Department of Agriculture, Forest Service, Pacific Southwest Research Station.
Brasier, C. (2008). Phytophthora ramorum + P. kernoviae = international biosecurity failure. In S. J. Frankel, J. T. Kliejunas, and K. M. Palmieri (Eds), Proceedings of the sudden oak death third science symposium (214, pp. 133–139). USDA Forest Service, Pacific Southwest Research Station, Albany, CA: US Department of Agriculture.
Brasier, C., & Kirk, S. (2004). Production of gametangia by Phytophthora ramorum in vitro. Mycological Research, 108(7), 823–827. https://doi.org/10.1017/s0953756204000565
Brasier, C., & Webber, J. (2010). Sudden larch death. Nature, 466, 824–825. https://doi.org/10.1038/466824a
Brown, A. V., & Brasier, C. M. (2007). Colonization of tree xylem by Phytophthora ramorum, P. kernoviae and other Phytophthora species. Plant Pathology, 56(2), 227–241. https://doi.org/10.1111/j.1365‐3059.2006.01511.x
CABI (Centre for Agriculture and Bioscience International). (2020). Phytophthora ramorum (Sudden Oak Death (SOD)). https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.40991 (accessed 2024‐02‐09).
Cave, G. L., Randall‐Schadel, B., & Redlin, S. C. (2008). Risk analysis for Phytophthora ramorum Werres, de Cock & Man in't Veld, causal agent of sudden oak death, ramorum leaf blight, and ramorum dieback. US Department of Agriculture, Animal and Plant Health Inspection Service, Raleigh, NC. 88 pp.
Davidson, J. M., Rizzo, D. M., Garbelotto, M., Tjosvold, S., & Slaughter, G. W. (2002). Phytophthora ramorum and sudden oak death in California: II. Transmission and survival. In R. B. Standiford, D. McCreary, K. L. Purcell (Eds.), Proceedings of the fifth symposium on oak woodlands: Oaks in California's challenging landscape (184, pp. 741–749). San Diego, California, US Department of Agriculture, Forest Service, Pacific Southwest Research Station.
Davidson, J. M., Werres, S., Garbelotto, M., Hansen, E. M., & Rizzo, D. M. (2003). Sudden oak death and associated diseases caused by Phytophthora ramorum. Plant Health Progress, 4(1), 12. https://doi.org/10.1094/php‐2003‐0707‐01‐dg
Davidson, J. M., Wickland, A. C., Patterson, H. A., Falk, K. R., & Rizzo, D. M. (2005). Transmission of Phytophthora ramorum in mixed‐evergreen forest in California. Phytopathology, 95, 587–596. https://doi.org/10.1094/phyto‐95‐0587
DEFRA (Department for Environment, Food and Rural Affairs). (2008). Consultation on future management of risks from Phytophthora ramorum and Phytophthora kernoviae. DEFRA and Forestry Commission, the UK, 24 pp.
DEFRA (Department for Environment, Food and Rural Affairs). (2015). FERA list of natural hosts for Phytophthora ramorum with symptom and location. DEFRA and Forestry Commission, the UK, 11 pp. https://planthealthportal.defra.gov.uk/pests‐and‐diseases/high‐profile‐pests‐and‐diseases/phytophthora/
DEFRA (Department for Environment, Food and Rural Affairs). (2022). UK Risk Register Details for Phytophthora ramorum. https://planthealthportal.defra.gov.uk/pests‐and‐diseases/uk‐plant‐health‐risk‐register/viewPestRisks.cfm?cslref=23022 (accessed 2024‐02‐09).
EFSA PLH Panel (EFSA Panel on Plant Health). (2011). Scientific Opinion on the Pest Risk Analysis on Phytophthora ramorum prepared by the FP6 project RAPRA. EFSA Journal, 9(6), 2186. https://doi.org/10.2903/j.efsa.2011.2186
Elliot, M., Meagher, T. R., Harris, C., Searle, K., Purse, B. V., & Schlenzig, A. (2013). The epidemiology of Phytophthora ramorum and P. kernoviae at two historic gardens in Scotland. In S. J. Frankel, J. T. Kliejunas, K. M. Palmieri, and J. M. Alexander (Eds.), Sudden oak death fifth science symposium (pp. 23–32). Albany, CA, USA: US Department of Agriculture, Forest Service, Pacific Southwest Research Station.
Englander, L., Browning, M., & Tooley, P. W. (2006). Growth and sporulation of Phytophthora ramorum in vitro in response to temperature and light. Mycologia, 98(3), 365–373. https://doi.org/10.3852/mycologia.98.3.365
EPPO (European and Mediterranean Plant Protection Organization). (2013). Pest risk management for Phytophthora kernoviae and Phytophthora ramorum. EPPO, Paris. http://www.eppo.int/QUARANTINE/Pest_Risk_Analysis/PRA_intro.htm
EPPO (European and Mediterranean Plant Protection Organization). (2024a). EPPO A2 List of pests recommended for regulation as quarantine pests, version 2023–09. https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list (accessed 2024‐02‐09).
EPPO (European and Mediterranean Plant Protection Organization). (2024b). Phytophthora ramorum (PHYTRA), Categorization. https://gd.eppo.int/taxon/PHYTRA/categorization (accessed 2024‐02‐09).
EPPO (European and Mediterranean Plant Protection Organization). (2024c). Phytophthora ramorum (PHYTRA), Distribution. https://gd.eppo.int/taxon/PHYTRA/distribution (accessed 2024‐02‐09).
EPPO (European and Mediterranean Plant Protection Organization). (2024d). Phytophthora ramorum (PHYTRA), Host plants. https://gd.eppo.int/taxon/PHYTRA/hosts (accessed 2024‐02‐09).
Erwin, D. C., & Ribeiro, O. K. (1996). Phytophthora diseases worldwide. St. Paul, Minnesota: APS Press, American Phytopathological Society, 562 pp.
EUROPHYT (European Union Notification System for Plant Health Interceptions). (2024). https://food.ec.europa.eu/plants/plant‐health‐and‐biosecurity/europhyt_en (accessed 2024‐02‐09).
Farr D. F., & Rossman, A. Y. (2024). Fungal Databases, U.S. National Fungus Collections, ARS, USDA. https://fungi.ars.usda.gov/ (accessed 2024‐02‐09).
Grünwald, N. J., Goss, E. M., & Press, C. M. (2008). Phytophthora ramorum: a pathogen with a remarkably wide host range causing sudden oak death on oaks and ramorum blight on woody ornamentals. Molecular Plant Pathology, 9(6), 729–740. https://doi.org/10.1111/j.1364‐3703.2008.00500.x
Grünwald, N. J., Goss, E. M., Ivors, K., Garbelotto, M., Martin, F. N., Prospero, S., Hansen, E., Bonants, P. J. M., Hamelin, R. C., Chastagner, G., Werres, S., Rizzo, D. M., Abad, G., Beales, P., Bilodeau, G. J., Blomquist, C. L., Brasier, C., Brière, S. C., Chandelier, A., Davidson, J. M., Denman, S., Elliott, M., Frankel, S. J., Goheen, E. M., de Gruyter, H., Heungens, K., James, D., Kanaskie, A., McWilliams, M. G., Man in ‘t Veld, W., Moralejo, E., Osterbauer, N. K., Palm, M. E., Parke, J. L., Perez Sierra, A. M., Shamoun, S. F., Shishkoff, N., Tooley, P. W., Vettraino, A. M., Webber, J., & Widmer, T. L. (2009). Standardizing the nomenclature for clonal lineages of the sudden oak death pathogen, Phytophthora ramorum. Phytopathology, 99(7), 792–795.
Jung, T., Jung, M. H., Webber, J. F., Kageyama, K., Hieno, A., Masuya, H., Uematsu, S., Pérez‐Sierra, A., Harris, A. R., Forster, J., Rees, H., Scanu, B., Patra, S., Kudláček, T., Janoušek, J., Corcobado, T., Milenković, I., Nagy, Z., Csorba, I., Bakonyi, J., & Brasier, C. M. (2021). The destructive tree pathogen Phytophthora ramorum originates from the laurosilva forests of East Asia. Journal of Fungi, 7(3), 226, 32 pp. https://doi.org/10.3390/jof7030226
King, K. M., Harris, A. R., & Webber, J. F. (2015). In planta detection used to define the distribution of the European lineages of Phytophthora ramorum on larch (Larix) in the UK. Plant Pathology, 64(5), 1168–1175.
Lilja, A., Rytkönen, A., Kokkola, M., Parikka, P., & Hantula, J. (2007). First report of Phytophthora ramorum and P. inflata in ornamental rhododendrons in Finland. Plant Disease, 91(8), 1055–1055.
Parke, J. L., & Lewis, C. (2007). Root and stem infection of Rhododendron from potting medium infested with Phytophthora ramorum. Plant Disease, 91, 1265–1270. https://doi.org/10.1094/pdis‐91‐10‐1265
Poimala, A., & Lilja, A. (2013). NOBANIS – Invasive Alien Species Fact Sheet – Phytophthora ramorum. From: Online Database of the European Network on Invasive Alien Species. 14 pp. https://www.nobanis.org/globalassets/speciesinfo/p/phytophthora‐ramorum/phytophthora_ramorum.pdf
Rizzo, D. M., Garbelotto, M., & Hansen, E. M. (2005). Phytophthora ramorum: integrative research and management of an emerging pathogen in California and Oregon forests. Annual Review of Phytopathology, 43(1), 13.1–13.27. https://doi.org/10.1146/annurev.phyto.42.040803.140418
Roubtsova, T. V., & Bostock, R. M. (2009). Episodic abiotic stress as a potential contributing factor to onset and severity of disease caused by Phytophthora ramorum in Rhododendron and Viburnum. Plant Disease, 93(9), 912–918. https://doi.org/10.1094/pdis‐93‐9‐0912
Sansford, C. E., Inman, A. J., Baker, R., Brasier, C., Frankel, S., de Gruyter, J., Husson, C., Kehlenbeck, H., Kessel, G., Moralejo, E., Steeghs, M., Webber, J., & Werres, S. (2009). Report on the risk of entry, establishment, spread and socio‐economic loss and environmental impact and the appropriate level of management for Phytophthora ramorum for the EU. Deliverable Report 28. EU Sixth Framework Project RAPRA. 310 pp.
Shishkoff, N. (2007). Persistence of Phytophthora ramorum in soil mix and roots of nursery ornamentals. Plant Disease, 91(10), 1245–1249. https://doi.org/10.1094/pdis‐91‐10‐1245
Thompson, C. H., McCartney, M. M., Roubtsova, T. V., Kasuga, T., Ebeler, S. E., Davis, C. E., & Bostock, R. M. (2021). Analysis of volatile profiles for tracking asymptomatic infections of Phytophthora ramorum and other pathogens in Rhododendron. Phytopathology, 111(10), 1818–1827. https://doi.org/10.1094/phyto‐10‐20‐0472‐r
Thomsen, I. M., Alsenius, B., Flø, D., Krokene, P., Wendell, P. H. M., Wright, S., Sæthre, M. G., Børve, J., Magnusson, C., Nicolaisen, M., Nybakken, L., & Stenberg, J. A. (2023). Updated pest risk assessment of Phytophthora ramorum in Norway. Scientific Opinion of the Panel on Plant Health of the Norwegian Scientific Committee for Food and Environment. Norwegian Scientific Committee for Food and Environment (VKM), Oslo, Norway. 88 pp. https://nmbu.brage.unit.no/nmbu‐xmlui/handle/11250/3098330
TRACES‐NT. (2024). TRAde Control and Expert System. https://webgate.ec.europa.eu/tracesnt (accessed 2024‐02‐09).
USDA (United States Department of Agriculture). (2023). Risk of Phytophthora ramorum to the United States. Version 2, 2023. 60 pp.
Van Poucke, K., Franceschini, S., Webber, J., Vercauteren, A., Turner, J. A., Mccracken, A. R., Heungens, K., & Brasier, C. (2012). Discovery of a fourth evolutionary lineage of Phytophthora ramorum: EU2. Fungal Biology, 116, 1178–1191. https://doi.org/10.1016/j.funbio.2012.09.003
Webber, J. F., Mullett, M., & Brasier, C. M. (2010). Dieback and mortality of plantation Japanese larch (Larix kaempferi) associated with infection by Phytophthora ramorum. New Disease Reports, 22(19), 2044–0588.
A.4. Thaumetopoea processionea
A.4.1. Organism information
| Taxonomic information |
Current valid scientific name: Thaumetopoea processionea Synonyms: Cnethocampa processionea Name used in the EU legislation: Thaumetopoea processionea Order: Lepidoptera Family: Notodontidae Common name: Oak processionary moth (OPM), oak processionary caterpillar Name used in the Dossier: Thaumetopoea processionea |
|
| Group | Insects | |
| EPPO code | THAUPR | |
| Regulated status |
Thaumetopoea processionea is listed in the Annex III of Regulation (EU) 2019/2072 as protected zone quarantine pest for Ireland. It is protected zone quarantine pest in the UK and included in A1 lists for Argentina and Türkiye (EPPO, 2024a). The Panel noted that the species is present in Türkiye (Groenen & Meurisse, 2012). |
|
| Pest status in the UK |
T. processionea is present in the UK with restricted distribution. It is a species under official control, currently found in the London area and in the Southeast of England (EPPO, 2024b; Forestry Commission, 2024a). According to Suprunenko et al. (2022) the eradication of T. processionea from the UK territory is ‘no longer considered a feasible option’. In 2006 it was found breeding at three separate sites in southwest London (Townsend, 2006). There were other previous records of the moth in the UK (south coast from Cornwall to Essex, islands Jersey and Guernsey), however, these records refer to immigrant moths caught in traps (Foster, 1983; Riley, 1985, 1987; Townsend, 2006). |
|
| Pest status in the EU |
T. processionea is a native European species reported to be present in 22 EU member states; it is absent from Estonia, Finland, Latvia, Lithuania and Malta (EPPO, 2024c; GBIF, 2024). In Ireland it was introduced in 2020 and eradicated in 2021. In June 2023 the NPPO of Ireland has newly detected the pest in the municipality of Castleknock and eradication measures have been immediately applied. The current pest status for Ireland declared by NPPO is ‘under determination’ whereas the current pest situation evaluated by EPPO is transient (EPPO, 2024d). According to Groenen and Meurisse (2012) the discontinuous occurrence of T. processionea in central‐northern Europe in the last two centuries, and its recent massive reappearance in north‐western Europe, are due to long‐term population fluctuations rather than range expansion. |
|
| Host status on Betula pendula and B. pubescens |
No information was found on whether B. pendula and B. pubescens are hosts for T. processionea. Stigter et al. (1997) reports Betula as an occasionalhost of T. processionea in the Netherlands. Moreover, according to Evans (2008) and Baker (2009) Betula is a host or occasional host to T. processionea. |
|
| PRA information | Available Pest Risk Assessment:
|
|
| Other relevant information for the assessment | ||
| Biology |
T. processionea is native to southern and central Europe, where it is more abundant and widespread in warm and sunny sites; in central and western Europe its presence is mainly dependent on population fluctuations which can be determined by aridity and climate change (Csóka et al., 2018; Groenen & Meurisse, 2012). The moth is also present in Türkiye and in the Middle East (Syria, Lebanon, Jordan, Israel) (Battisti et al., 2015; Basso et al., 2017; CABI, 2024; Groenen & Meurisse, 2012). T. processionea has four life stages: egg, larva (six instars), pupa and adult; it is a univoltine species, overwintering as 1st instar larva, but at egg stage too (CABI, 2024; Forestry Commission, 2024b; Zielonka, 2020). Adults, 25–35 mm wingspan, fly from July to September and can survive 4–10 days. Females lay 30–200 eggs, occasionally up to 300 (CABI, 2024), which are 2 mm long. The eggs are laid in batches on small branches of oaks (3.5–10 mm diameter), more rarely on other hosts (Battisti et al., 2015). In autumn 1st instar larvae are found within the eggs; eggs and larvae are known to withstand up to −30°C, and a 90% rate of survival of overwintering eggs is observed after severe winters (Baker et al., 2009; Battisti et al., 2015). Egg hatching in April–May is usually well synchronised with oak bud flushing. The larval stage can last 60–70 days. Larvae feed on foliage gregariously from April to July and build a silky nest for each of the instars (CABI, 2024); however, a large bag‐shaped nest incorporating hairs, frass and silk, is built only at 5th–6th larval stage in the medium‐lower part of the trunk. The 35–40 mm mature caterpillars rest in the nest during the day and move in nose‐to‐tail processions during the night in search of food. Larvae from 3rd instar onwards develop urticating hairs on the dorsal part of abdomen (CABI, 2024; EPPO, 2024e; Zielonka, 2020). In the UK, mature larvae pupate inside the nests from June to early September and adult flight can be normally observed from end of July to late September (Forestry Commission, 2024b). Natural dispersal of T. processionea is through larval processions and adult flight. Larvae can move in processions only to short distances, but adults are good flyers (50–100 km/year for males and 5–20 km/year for females); windborne spread of adults is also likely (Baker et al., 2009; EPPO, 2024e). Males are known to be able to fly over the Channel from France to southern England; this is considered unlikely for females, which are heavier (Battisti et al., 2015; EPPO, 2024e; Evans, 2008). In the UK, T. processionea has recently increased its expansion rate, passing from 1.66 km/year in 2006–2014 to 6.17 km/year in 2015–2019 (Suprunenko et al., 2022). The spread of T. processionea can also be human supported, mostly via trading of plants for planting carrying eggs, larvae and pupae. Cut branches and round wood with bark are considered pathways of lesser importance (Baker et al., 2009; EPPO, 2024e; Evans, 2008). T. processionea is both an important defoliating insect for oak species and a threat to human and domestic animal health. Marzano et al. (2020) provide a useful summary of how the multi‐face OPM problem is currently felt by people and managers in the UK. The impact of T. processionea on forest health is variable: it is considered a minor pest for oak forests in Ukraine, Romania, Hungary, Slovenia; severe damage was instead reported from Germany, Italy, France, Belgium and Spain (Baker et al., 2009). In western Europe (Belgium, the Netherlands) and in the UK, the pest is mainly harmful to urban and road trees, as well as to amenity oak trees in parks, forest edges and countryside hedgerows (Battisti et al., 2015). Both in canopied stands and open forests, oaks weakened after severe defoliation by the T. processionea become more susceptible to secondary pests as buprestid beetles, bark and ambrosia beetles or root rot fungi. T. processionea may be hence considered a contributing factor in the oak decline, also resulting in loss of biodiversity (Baker et al., 2009; CABI, 2024). No information was found about the impact of T. processionea on Betula. Impact on human health may be relevant mostly in urban areas, due to the severe pseudo‐allergenic reactions caused by the contact of urticating hairs released by the larvae with skin, eyes and respiratory system. A good synthesis on health effects of T. processionea is provided by Rahlenbeck and Utikal (2015). Urticating hairs released by larvae spread by air currents also from nests, exuviae, pupal cases and may remain active in the soil or in the litter for several years lengthening the social impact of the species (Baker et al., 2009). |
|
| Symptoms | Main type of symptoms |
Main symptoms caused by larvae of T. processionea on oaks are skeletonisation of leaves and defoliation; presence of silken nests mainly on the lower branches and the lower part of the trunk; processions of caterpillars on the branches and trunks; egg batches in rows covered by scales, mostly on 1–2 years‐old twigs. No specific symptoms on Betula are known. Symptoms on humans and animals due to urticating hairs are skin rash, eye irritation, sore throat and breathing difficulty. |
| Presence of asymptomatic plants | No information on the presence of asymptomatic plants was found. | |
| Confusion with other pests | T. processionea is one of 15 species belonging to the genus Thaumetopoea worldwide, recently revised by Basso et al. (2017). The species is easily identified by both morphological features of adults, and features and host plants of larvae (it is the sole Thaumetopoea feeding on Quercus sp.) so that no confusion with other similar species is possible. | |
| Host plant range |
T. processionea is a specialist herbivore feeding on oaks in Europe (Damestoy, 2019). Quercus species known to be hosts of T. processionea are Quercus boissieri, Q. calliprinos, Q. cerris, Q. frainetto, Q. infectoria, Q. ilex, Q. palustris, Q. petraea, Q. pubescens, Q. pyrenaica, Q. robur, Q. × turneri (Baker et al., 2009; DEFRA, 2024; EPPO, 2024f; EUROPHYT, 2024). Occasional hosts during outbreaks on which are Acacia, Betula, Carpinus, Castanea, Corylus, Crataegus, Juglans, Fagus, Pistacia, Robinia and Sorbus (Baker et al., 2009; CABI, 2024; EPPO, 2024f; Evans, 2008; Stigter et al., 1997). On these trees larvae were found to feed but without complete development of the life cycle. Only on Fagus they can reach the pupal stage (EPPO, 2024e, 2024f; Stigter et al., 1997). |
|
| Reported evidence of impact | T. processionea is an EU protected zone quarantine pest. | |
| Evidence that the commodity is a pathway | Although there are no reports of Betula pendula or B. pubescens infested by T. processionea, Betula is reported bearing dispersed feeding larvae during outbreaks on major hosts. Major hosts of T. processionea (Quercus spp.) are present both in the nurseries and in the surroundings of the nurseries. Therefore, a spillover of larvae may occur making the association with the commodity possible particularly if plants are exported with leaves. | |
| Surveillance information | T. processionea is quarantine pest for which Great Britain is a pest‐free area (excluding the local authority areas in infested zone) (Dossier Section 5.2). | |
A.4.2. Possibility of pest presence in the nursery
A.4.2.1. Possibility of entry from the surrounding environment
T. processionea is present in the UK territory with restricted distribution in London area and the Southeast of England (EPPO, 2024b; Forestry Commission, 2024a).
Adult moths have considerable spreading capacities (50–100 km/year for males and 5–20 km/year for females); in the UK, the pest has strongly increased its expansion rate, passing from 1.66 km/year in 2006–2014 to 6.17 km/year in 2015–2019 (Suprunenko et al., 2022).
T. processionea breeds on Quercus species. On Fagus the mature larvae can complete the development according to Stigter et al. (1997) but oviposition and young larvae were never observed. The major host Quercus and other plant species that larvae have been found feeding like Betula spp., Corylus spp., Crataegus spp., Fagus spp., are present within 2 km from the nurseries (Dossier Sections 1.1, 1.2 and 5.1).
Uncertainties
-
–
The possibility of presence of the pest in the surrounding area of nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for T. processionea to enter the nurseries from surrounding environment. In the surrounding area, suitable hosts are present and flying adult moths can easily reach the nurseries.
A.4.2.2. Possibility of entry with new plants/seed
The starting materials are either seeds, seedlings or shoots/buds when grafted plants are produced. Seeds are certified and coming from the UK. Seedlings are also certified and are either from the UK or the EU (the Netherlands) (Dossier Sections 1.1 and 1.2).
In addition to B. pendula and B. pubescens plants, the nurseries also produce other plants (Dossier Sections 3.1, 3.2 and 5.1). Out of them, there are major hosts for the pest (Quercus spp.) and occasional hosts (such as Acacia spp., Carpinus spp., Castanea spp., Corylus spp., Crataegus spp., Fagus spp., Juglans spp., Robinia spp. and Sorbus spp). However, there is no information on how and where the plants are produced. Therefore, if the plants are first produced in another nursery, the pest could possibly travel with them.
In the nurseries, virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) is used as a growing media (Dossier Sections 1.1 and 1.2). The growing media is certified and heat‐treated by commercial suppliers during production to eliminate pests and diseases (Dossier Sections 1.1 and 1.2). Soil and growing media are not pathways for T. processionea.
Uncertainties
-
–
No information is available on the origin of plants (Quercus spp. Fagus spp. and Fagus sylvatica and other plants included in the host range of T. processionea) used for plant production in the area of the nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nurseries via new seedlings of Quercus spp., Fagus spp., Fagus sylvatica (and other plants that are hosts for the pest) used for plant production in the area. The entry of the pest with seeds and the growing media the Panel considers as not possible.
A.4.2.3. Possibility of spread within the nursery
Betula plants are either grown in containers (cells, pots, tubes, etc.) or in field. Cell‐grown trees may be grown in greenhouses, however most plants will be field grown or field grown in containers (Dossier Sections 1.1 and 1.2). One of the nurseries have mother plants of B. pendula (Dossier Sections 1.1 and 1.2), which could serve as a reservoir of the pest.
The pest can infest other suitable plants (such as Quercus spp., Fagus spp., etc.) present within the nurseries (Dossier Sections 3.1 and 3.2).
Thaumetopoea processionea can spread within the nurseries by movement of larvae, adult flight and infested plant material.
Uncertainties
-
–
None.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pest within the nurseries is possible both by movement of infested plant material, larvae and flight of adult moths.
A.4.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database there are no records of notification of Betula plants for planting due to the presence of T. processionea between the years 1995 and January 2024 (EUROPHYT, 2024; TRACES‐NT, 2024).
In the same period, there are 88 records of notification of Quercus plants for planting (Quercus cerris, Q. frainetto, Q. petraea, Q. robur, Q. × turneri) from the Netherlands, Germany and Belgium, all for plants intended for planting, already planted (EUROPHYT, 2024; TRACES‐NT, 2024).
A.4.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on T. processionea is provided. The description of the risk mitigation measures currently applied in the UK is provided in the Table 8.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
The registration and the release of the UK plant passport should be enough to warrant pest‐free plant material for a protected zone quarantine pest in the UK.
Uncertainties
|
| 2 | Physical separation | No | Not relevant, as the production is not carried out in separate areas, the possibility that the pest can move from the outside to the nurseries and from one tree species to another within the nurseries is concrete. |
| 3 | Certified plant material | Yes |
The use of certified material should be enough to warrant pest‐free status.
Uncertainties
|
| 4 | Growing media | No | Not relevant. The pest is not affected by the growing medium as in the nurseries all the stages develop above ground. |
| 5 | Surveillance, monitoring and sampling | Yes |
Regular surveys are carried out during the production by visual inspection of the plants. Any report of a quarantine pest is provided.
Uncertainties
|
| 6 | Hygiene measures | No | Weeding and disinfection are not relevant for this pest. |
| 7 | Removal of infested plant material | Yes |
The removal of infested plants at the larval stage will have a positive effect. Egg masses are not expected on Betula.
Uncertainties
|
| 8 | Irrigation water | No | Water is not relevant for this pest. |
| 9 | Application of pest control measures | Yes |
The pest is easy to control at the larval stage and being a quarantine pest, its presence must be reported and measures taken. However, with the exception of egg parasitoids and other generalist enemies feeding on eggs, the egg masses are not susceptible to any crop protection method. No treatments available against the moths.
Uncertainties
|
| 10 | Measures against soil pests | No | Soil is not relevant for this pest. |
| 11 | Inspections and management of plants before export | Yes |
Inspections carried out before export will be visual and would be enough to warrant that commodities are free of larvae.
Uncertainties
|
| 12 | Separation during transport to the destination | Yes |
The separation of the plants during the transport would reduce the possibility that larvae are moving among plants if the transport happens when green leaves are occurring between April and August.
Uncertainties
|
A.4.5. Overall likelihood of pest freedom for bare root plants
The scenarios as well as the values were taken from the Scientific opinion on Corylus avellana from the UK (EFSA PLH Panel, 2024) because the similarity of the host suitability, of the commodities, of the production systems and on the nurseries and surroundings.
A.4.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare root plants
No major hosts in the surrounding of the nurseries so no possibility of spillover on the nursery plants of Betula.
A.4.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare root plants
Presence of major hosts in the surrounding of the nurseries with high population of the moth leading to possibility of spillover on the nursery plants of Betula.
A.4.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare root plants (Median)
Median skewed to the left because of the low probability that an outbreak is occurring on oak trees close to the nurseries, and that larvae can spillover on the nursery plants of Betula.
A.4.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Highest uncertainty on both sides of the median because of the scarce or missing information about the occurrence of oak trees with high density of the oak processionary moth in the surroundings of the nurseries.
A.4.5.5. Elicitation outcomes of the assessment of the pest freedom for Thaumetopoea processionea on bare root plants
The following Tables show the elicited and fitted values for pest infestation (Table A.23) and pest freedom (Table A.24).
TABLE A.23.
Elicited and fitted values of the uncertainty distribution of pest infestation by Thaumetopoea processionea per 10,000 plants/bundles.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 1 | 3 | 5 | 12 | ||||||||||
| EKE | 0.0204 | 0.0604 | 0.138 | 0.317 | 0.594 | 0.987 | 1.43 | 2.53 | 4.00 | 4.97 | 6.22 | 7.64 | 9.28 | 10.6 | 12.1 |
Note: The EKE results is the BetaGeneral (0.84634, 3.4138, 0, 16.8) distribution fitted with @Risk version 7.6.
Based on the numbers of estimated infested bundles the pest freedom was calculated (i.e. = 10,000 – number of infested plants/bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.24.
TABLE A.24.
The uncertainty distribution of plants free of Thaumetopoea processionea per 10,000 plants/bundles calculated by Table A.23.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9988 | 9995 | 9998 | 9999 | 10,000 | ||||||||||
| EKE results | 9988 | 9989 | 9991 | 9992 | 9994 | 9995 | 9996 | 9997 | 9998.6 | 9999.0 | 9999.4 | 9999.7 | 9999.86 | 9999.94 | 9999.98 |
Note: The EKE results are the fitted values.
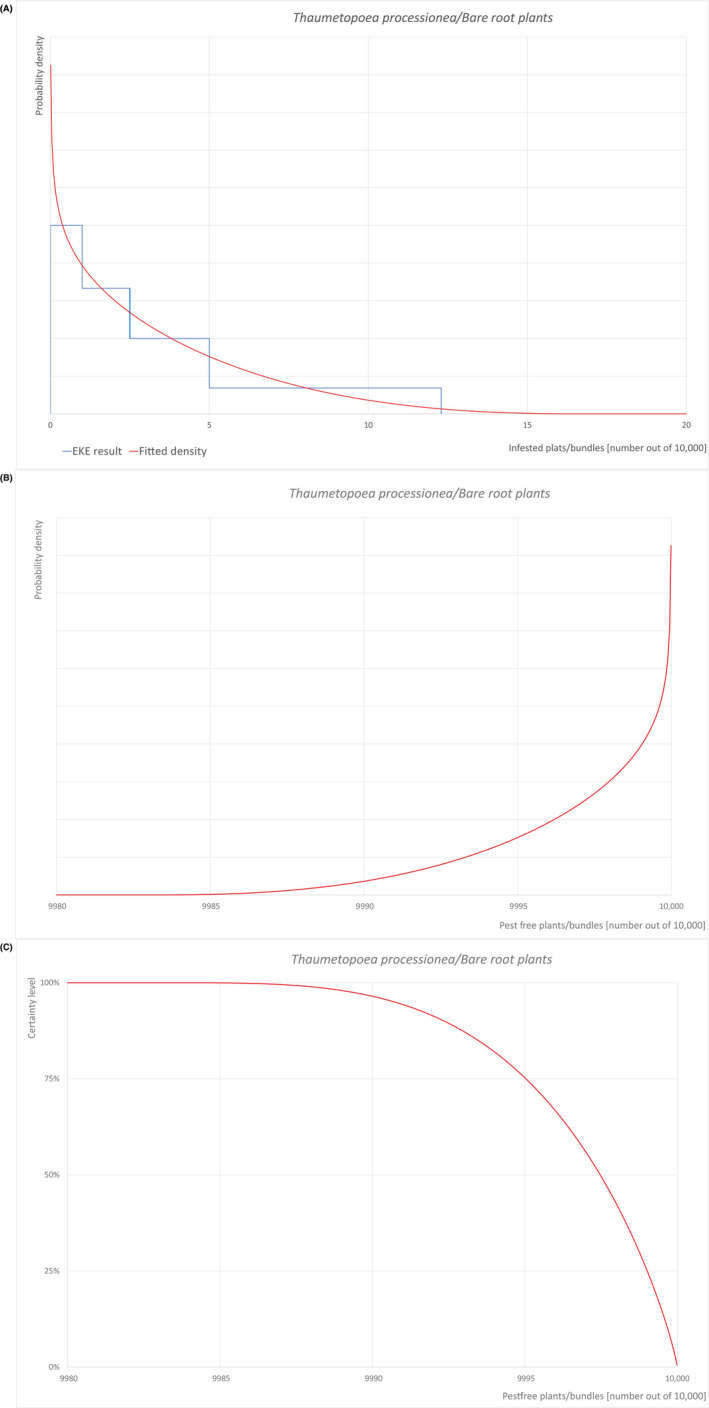
FIGURE A.12 (A) Elicited uncertainty of pest infestation per 10,000 plants/bundles of graftwood/budwood (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (B) uncertainty of the proportion of pest‐free plants/bundles per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (C) descending uncertainty distribution function of pest infestation per 10,000 plants/bundles.
A.4.6. Overall likelihood of pest freedom for plants in pots
The scenarios as well as the values were taken from the Scientific opinion on Corylus avellana from the UK (EFSA PLH Panel, 2024) because the similarity of the host suitability, of the commodities, of the production systems and on the nurseries and surroundings.
A.4.6.1. Reasoning for a scenario which would lead to a reasonably low number of infested plants in pots
No oak trees in the surrounding of the nurseries so no possibility of spillover on the nursery plants of Betula.
A.4.6.2. Reasoning for a scenario which would lead to a reasonably high number of infested plants in pots
Presence of oak trees in the surrounding of the nurseries with high density of the moth leading to possibility of spillover on the nursery plants of Betula.
A.4.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested plants in pots (Median)
Median skewed to the left because of the low probability that an outbreak is occurring on oak trees close to the nurseries, and that larvae can move on the nursery plants of Betula.
A.4.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Highest uncertainty on both sides of the median because of the scarce or missing information about the occurrence of oak trees with high density of the oak processionary moth in the surroundings of the nurseries.
A.4.6.5. Elicitation outcomes of the assessment of the pest freedom for Thaumetopoea processionea on plants in pots
The following Tables show the elicited and fitted values for pest infestation (Table A.25) and pest freedom (Table A.26).
TABLE A.25.
Elicited and fitted values of the uncertainty distribution of pest infestation by Thaumetopoea processionea per 10,000 plants/bundles.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 1 | 3 | 5 | 12 | ||||||||||
| EKEresults | 0.0204 | 0.0604 | 0.138 | 0.317 | 0.594 | 0.987 | 1.43 | 2.53 | 4.00 | 4.97 | 6.22 | 7.64 | 9.28 | 10.6 | 12.1 |
Note: The EKE results is the BetaGeneral (0.84634, 3.4138, 0, 16.8) distribution fitted with @Risk version 7.6.
Based on the numbers of estimated infested bundles the pest freedom was calculated (i.e. = 10,000 – number of infested plants/bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.26.
TABLE A.26.
The uncertainty distribution of plants free of Thaumetopoea processionea per 10,000 plants/bundles calculated by Table A.25.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 9988 | 9995 | 9998 | 9999 | 10,000 | ||||||||||
| EKE results | 9988 | 9989 | 9991 | 9992 | 9994 | 9995 | 9996 | 9997 | 9998.6 | 9999.0 | 9999.4 | 9999.7 | 9999.86 | 9999.94 | 9999.98 |
Note: The EKE results are the fitted values.
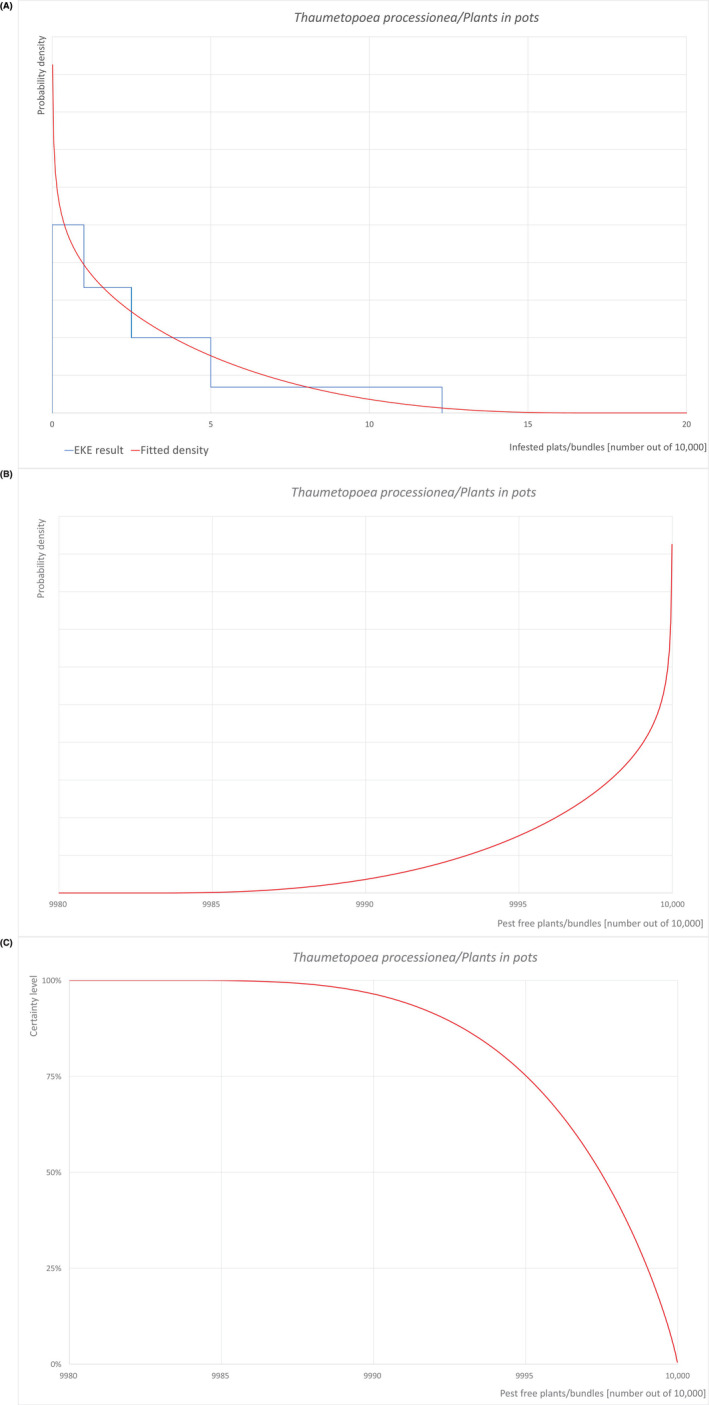
FIGURE A.13 (A) Elicited uncertainty of pest infestation per 10,000 plants/bundles of plants in pots (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (B) uncertainty of the proportion of pest‐free plants/bundles per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (C) descending uncertainty distribution function of pest infestation per 10,000 plants/bundles.
A.4.7. Overall likelihood of pest freedom for specimen trees
A.4.7.1. Reasoning for a scenario which would lead to a reasonably low number of infested specimen trees
No oak trees in the surrounding of the nurseries so no possibility of larvae spillover on the nursery plants of Betula.
A.4.7.2. Reasoning for a scenario which would lead to a reasonably high number of infested specimen trees
Presence of oak trees in the surrounding of the nurseries with high density of the moth leading to possibility of spillover on the nursery plants of Betula.
A.4.7.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested specimen trees (Median)
Median skewed to the left because of the low probability that an outbreak is occurring on oak trees close to the nurseries, and that larvae can move on the nursery plants of Betula.
A.4.7.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Highest uncertainty on both sides of the median because of the scarce or missing information about the occurrence of oak trees with high density of the oak processionary moth in the surroundings of the nurseries.
A.4.7.5. Elicitation outcomes of the assessment of the pest freedom for Thaumetopoea processionea on specimen trees
The following Tables show the elicited and fitted values for pest infestation (Table A.27) and pest freedom (Table A.28).
TABLE A.27.
Elicited and fitted values of the uncertainty distribution of pest infestation by Thaumetopoea processionea per 10,000 plants.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0.0 | 3.5 | 7.0 | 11.0 | 25.0 | ||||||||||
| EKE results | 0.345 | 0.651 | 1.06 | 1.76 | 2.60 | 3.60 | 4.60 | 6.80 | 9.49 | 11.2 | 13.4 | 16.0 | 19.1 | 21.8 | 25.0 |
Note: The EKE results is the BetaGeneral (1.4832, 7.3195, 0, 47.5) distribution fitted with @Risk version 7.6.
Based on the numbers of estimated infested bundles the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.28.
TABLE A.28.
The uncertainty distribution of plants free of Thaumetopoea processionea per 10,000 plants calculated by Table A.27.
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 9975 | 9989 | 9993 | 9997 | 10,000 | ||||||||||
| EKE results | 9975 | 9978 | 9981 | 9984 | 9987 | 9989 | 9991 | 9993 | 9995 | 9996 | 9997 | 9998.2 | 9998.9 | 9999.3 | 9999.7 |
Note: The EKE results are the fitted values.
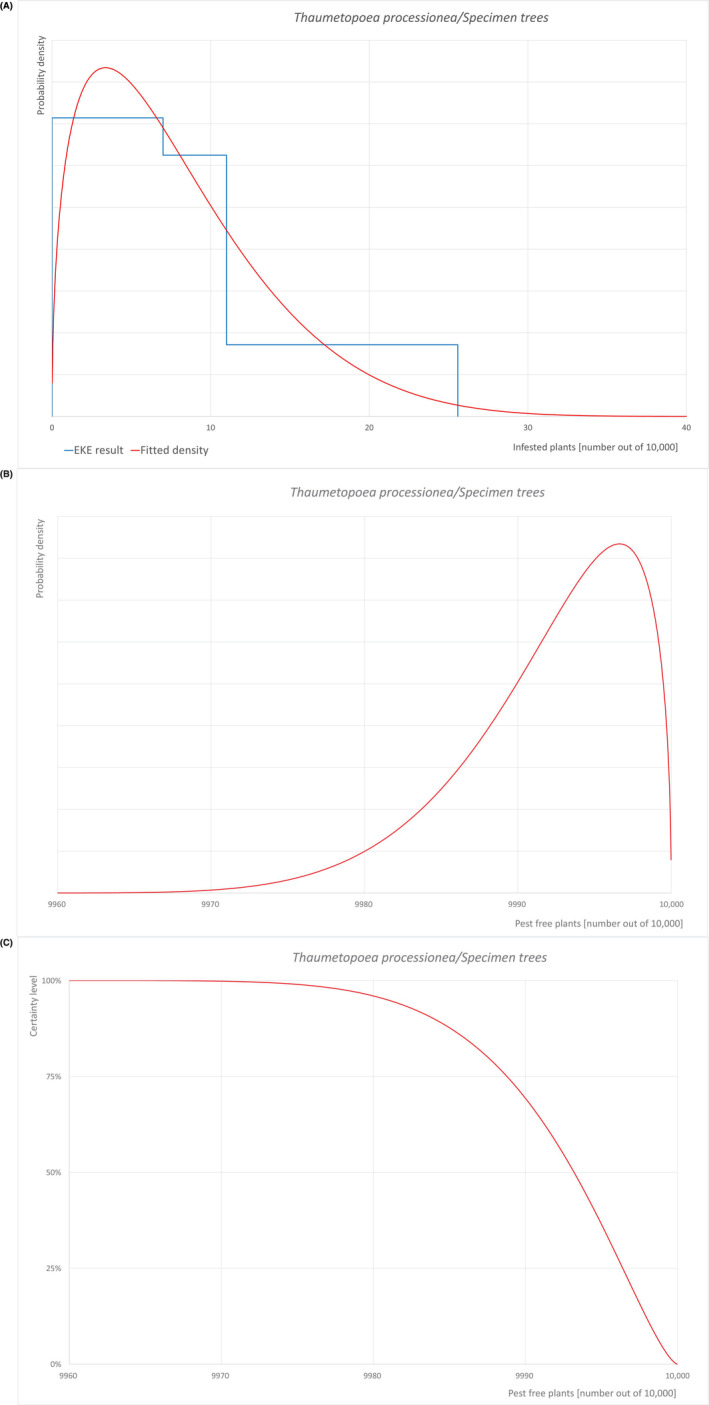
FIGURE A.14 (A) Elicited uncertainty of pest infestation per 10,000 plants of specimen trees (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (B) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (C) descending uncertainty distribution function of pest infestation per 10,000 plants.
A.4.8. Reference list
Baker, R., Caffier, D., Choiseul, J. W., De Clercq, P., Dormannnsné‐Simon, E., Gerowitt, B., Karadjova, O. E., Lövei, G., Lansink, A. O., Makowski, D., Manceau, C., Manici, L., Perdikis, D., Puglia, A. P., Schans, J., Schrader, G., Steffek, R., Strömberg, A., Tiilikkala, K., van Lenteren, J. C., & Vloutoglou, I. (2009). Scientific opinion of the Panel of Plant Health on a pest risk analysis on Thaumetopoea processionea L., the oak processionary moth, prepared by the UK and extension of its scope to the EU territory. The EFSA Journal, 491, 1–63. https://doi.org/10.2903/j.efsa.2009.1195
Basso, A., Negrisolo, E., Zilli, A., Battisti, A., & Cerretti, P. (2017). A total evidence phylogeny for the processionary moths of the genus Thaumetopoea (Lepidoptera: Notodontidae: Thaumetopoeinae). Cladistics, 33(6), 557–573. https://doi.org/10.7934/p2806
Battisti, A., Avci, M., Avtzis, D., Mohamed Lahbib, B. J., Berardi, L., Wahiba, B., Branco, M., Chakali G, Fels M. A. E. A. E., Frérot, B., Hódar, J., Ionescu‐Mălăncuş, I., Ipekdal, K., Larsson, S., Traian, M., Mendel, Z., Meurisse, N., Mirchev, P., Nemer, N., & Zamoum, M. (2015). Natural history of the processionary moths (Thaumetopoea spp.): new insights in relation to climate change. In A Roques (Ed.), Processionary moths and climate change: an update (pp. 15–81). Springer Dordrecht. https://doi.org/10.1007/978‐94‐017‐9340‐7_2
CABI (Centre for Agriculture and Bioscience International). (2024). Thaumetopoea processionea (oak processionary moth). https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.53502 (accessed 2024‐02‐12).
Csóka, G., Hirka, A., Szöcs, L., Móritz, N., Rasztovits, E., & Pödör, Z. (2018). Weather‐dependent fluctuations in the abundance of the oak processionary moth, Thaumetopoea processionea (Lepidoptera: Notodontidae). European Journal of Entomology, 115, 249–255. https://doi.org/10.14411/eje.2018.024
Damestoy, T. (2019). Interactions between oaks and the oak processionary moth, Thaumetopoea processionea L.: from trees to forest. Biodiversity and Ecology. Université de Bordeaux, 128 pp.
DEFRA (Department for Environment, Food and Rural Affairs). (2024). UK risk register details for Thaumetopoea processionea. https://planthealthportal.defra.gov.uk/pests‐and‐diseases/uk‐plant‐health‐risk‐register/viewPestRisks.cfm?cslref=7319 (accessed 2024‐02‐12).
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard, C., Baptista, P., Chatzivassiliou, E., Di Serio, F., Jaques Miret, J. A., Justesen, A. F., MacLeod, A., Magnusson, C. S., Milonas, P., Navas‐Cortes, J. A., Parnell, S., Potting, R., Reignault, P. L., Stefani, E., Thulke, H.‐H., Van der Werf, W., Vicent Civera, A. V., Yuen, J., Lucia Zappalà, L., Battisti, A., Mas, H., Rigling, D., Faccoli, M., Mikulová, A., Mosbach‐Schulz, O., Stergulc, F., Streissl, F., & Gonthier, P. (2024). Commodity risk assessment of Corylus avellana plants from the UK. EFSA Journal, 22(1), e8495. https://doi.org/10.2903/j.efsa.2024.8495
EPPO (European and Mediterranean Plant Protection Organization). (2024a). Thaumetopoea processionea (THAUPR), Categorization. https://gd.eppo.int/taxon/THAUPR/categorization (accessed 2024‐02‐12).
EPPO (European and Mediterranean Plant Protection Organization). (2024b). Thaumetopoea processionea (THAUPR). Distribution details in United Kingdom. https://gd.eppo.int/taxon/THAUPR/distribution/GB (accessed 2024‐02‐12).
EPPO (European and Mediterranean Plant Protection Organization). (2024c). Thaumetopoea processionea (THAUPR), Distribution. https://gd.eppo.int/taxon/THAUPR/distribution (accessed 2024‐02‐12).
EPPO (European and Mediterranean Plant Protection Organization). (2024d). New finding of Thaumetopoea processionea in Ireland. EPPO Reporting Service no. 06–2023. Num. article: 2023/135. https://gd.eppo.int/reporting/article‐7617 (accessed 2024‐02‐12).
EPPO (European and Mediterranean Plant Protection Organization). (2024e). Thaumetopoea processionea. EPPO datasheet. https://gd.eppo.int/taxon/THAUPR/datasheet (accessed 2024‐02‐12).
EPPO (European and Mediterranean Plant Protection Organization). (2024f). Thaumetopoea processionea (THAUPR), Hosts. https://gd.eppo.int/taxon/THAUPR/hosts (accessed 2024‐02‐12).
Evans, H. F. (2008). Oak processionary moth Pest Risk Analysis. Revision June 2008. Forest Research, Tree Health Division. 30 pp.
EUROPHYT (European Union Notification System for Plant Health Interceptions). (2024). https://food.ec.europa.eu/plants/plant‐health‐and‐biosecurity/europhyt_en (accessed 2024‐02‐09).
Forestry Commission. (2024a). Tools and Resources, Oak processionary moth (Thaumetopoea processionea). Edinburgh, UK: Forestry Commission, Plant Health Service. https://www.forestresearch.gov.uk/tools‐and‐resources/fthr/pest‐and‐disease‐resources/oak‐processionary‐moth‐thaumetopoea‐processionea/ (accessed 2024‐02‐12).
Forestry Commission. (2024b). Oak processionary moth (Thaumetopoea processionea) ‐ Life cycle. Edinburgh, UK: Forestry Commission, Plant Health Service. https://www.forestresearch.gov.uk/tools‐and‐resources/fthr/pest‐and‐disease‐resources/oak‐processionary‐moth‐thaumetopoea‐processionea/opm‐manual‐4‐biology‐and‐life‐cycle/ (accessed 2024‐02‐12).
Foster, A. P. (1983). Thaumetopoea processionea (linn.) (the Oak Processionary Moth): the imago recorded in Britain, together with other rare migrants from Cornwall. Entomologist's Record and Journal of Variation, 95, 216.
GBIF (Global Biodiversity Information Facility). (2024). GBIF BackBone Taxonomy. https://www.gbif.org/ (accessed 2024‐02‐12).
Groenen, F., & Meurisse, N. (2012). Historical distribution of Thaumetopoea processionea in Europe suggests recolonization instead of expansion. Agricultural and Forest Entomology, 14, 147–155. https://doi.org/10.1111/j.1461‐9563.2011.00552.x
Marzano, M., Ambrose‐Oji, B., Hall, C., & Moseley, D. (2020). Pests in the city: managing public health risks and social values in response to Oak Processionary Moth (Thaumetopoea processionea) in the United Kingdom. Forests, 11(1), 199. https://doi.org/10.3390/f11020199
Rahlenbeck, S., & Utikal, J. (2015). The oak processionary moth: a new health hazard? British Journal of General Practice, 65, 435–436. https://doi.org/10.3399/bjgp15x686341
Riley, A. M. (1985). Thaumetopoea processionea L.: Oak Processionary Moth on Guernsey. Entomologist's Record and Journal of Variation, 97, 110–111.
Riley, A. M. (1987). Further records of Thaumetopoea processionea L. (Oak Processionary Moth) (Lep.: Thaumetopoeidae) on Jersey. Entomologist's Record and Journal of Variation, 99, 225–226.
Stigter, H., Geraedts, W. H. J. M., & Spijkers, H. C. P. (1997). Thaumetopoea processionea in the Netherlands: Present status and management perspectives (Lepidoptera: Notodontidae). Proceedings of the Section Experimental and Applied Entomology of the Netherlands Entomological Society (N.E.V.), 3–16.
Suprunenko, Y. F., Castle, M. D., Webb, C. R., Branson, J., Hoppit, A., & Gilligan, C. A. (2022). Estimating expansion of the range of oak processionary moth (Thaumetopoea processionea) in the UK from 2006 to 2019. Agricultural and Forest Entomology, 10 pp. https://doi.org/10.1111/afe.12468
Townsend, M. (2006). An outbreak of the oak processionary moth Thaumetopoea processionea (L.) (Lep.: Thaumetopoeidae) in south‐west London. The Entomologist's Record and Journal of Variation, 118, 193.
TRACES‐NT. (2024). TRAde Control and Expert System. https://webgate.ec.europa.eu/tracesnt (accessed 2024‐02‐09).
Zielonka, M. (2020). Pest case studies ‐ On the oak processionary moth Thaumetopoea processionea (Lepidoptera: Thaumetopoeidae), Harper Adams University, 9 pp.
APPENDIX B. Web of Science All Databases Search String
B.1.
In the Table B.1, the search string for B. pendula used in Web of Science is reported. Totally, 1092 papers were retrieved. Titles and abstracts were screened, and 141 pests were added to the list of pests (see Appendix F).
TABLE B.1.
String for Betula pendula.
| Web of Science All databases |
TOPIC: “Betula pendula” OR “B. pendula” OR “Betula alba var. pendula” OR “Betula alba lusus pendula” OR “Betula alba var. pendula” OR “Betula verrucosa” OR “clump birch” OR “common birch” OR “European white birch” OR “silver birch” AND TOPIC: pathogen* OR pathogenic bacteria OR fung* OR oomycet* OR myce* OR bacteri* OR virus* OR viroid* OR insect$ OR mite$ OR phytoplasm* OR arthropod* OR nematod* OR disease$ OR infecti* OR damag* OR symptom* OR pest$ OR vector OR hostplant$ OR “host plant$” OR host OR “root lesion$” OR decline$ OR infestation$ OR damage$ OR symptom$ OR dieback* OR “die back*” OR “malaise” OR aphid$ OR curculio OR thrip$ OR cicad$ OR miner$ OR borer$ OR weevil$ OR “plant bug$” OR spittlebug$ OR moth$ OR mealybug$ OR cutworm$ OR pillbug$ OR “root feeder$” OR caterpillar$ OR “foliar feeder$” OR virosis OR viroses OR blight$ OR wilt$ OR wilted OR canker OR scab$ OR rot OR rots OR rotten OR “damping off” OR “damping‐off” OR blister$ OR “smut” OR mould OR mold OR “damping syndrome$” OR mildew OR scald$ OR “root knot” OR “root‐knot” OR rootknot OR cyst$ OR “dagger” OR “plant parasitic” OR “parasitic plant” OR “plant$parasitic” OR “root feeding” OR “root$feeding” NOT TOPIC: “winged seeds” OR metabolites OR *tannins OR climate OR “maple syrup” OR syrup OR mycorrhiz* OR “carbon loss” OR pollut* OR weather OR propert* OR probes OR spectr* OR antioxidant$ OR transformation OR RNA OR DNA OR “Secondary plant metabolite$” OR metabol* OR “Phenolic compounds” OR Quality OR Abiotic OR Storage OR Pollen* OR fertil* OR Mulching OR Nutrient* OR Pruning OR drought OR “human virus” OR “animal disease*” OR “plant extracts” OR immunological OR “purified fraction” OR “traditional medicine” OR medicine OR mammal* OR bird* OR “human disease*” OR biomarker$ OR “health education” OR bat$ OR “seedling$ survival” OR “anthropogenic disturbance” OR “cold resistance” OR “salt stress” OR salinity OR “aCER method” OR “adaptive cognitive emotion regulation” OR nitrogen OR hygien* OR “cognitive function$” OR fossil$ OR *toxicity OR Miocene OR postglacial OR “weed control” OR landscape NOT TOPIC: “Absidia cylindrospora” OR “Absidia glauca” OR “Absidia spinosa” OR “Acalitus calycophthirus” OR “Acalitus longisetosus” OR “Acalitus longisetus” OR “Acalitus rudis” OR “Acantharia sinensis” OR “Acanthohelicospora scopula” OR “Acanthosoma haemorrhoidale” OR “Acanthostigma scopulum” OR “Acaphylla acromius” OR “Achlya flavicornis” OR “Acleris emargana” OR “Acleris lipsiana” OR “Acleris literana” OR “Acleris logiana” OR “Acleris notana” OR “Acolium inquinans” OR “Acremoniella atra” OR “Acremonium apii” OR “Acremonium bacillisporum” OR “Acremonium charticola” OR “Acremonium diversisporum” OR “Acremonium felinum” OR “Acremonium fusidioides” OR “Acronicta aceris” OR “Acronicta alni” OR “Acronicta auricoma” OR “Acronicta euphorbiae” OR “Acronicta leporina” OR “Acronicta menyanthidis” OR “Acronicta psi” OR “Acronicta rumicis” OR “Acronicta tridens” OR “Actias luna” OR “Aculis leionotus” OR “Adoxophyes orana” OR “Aethalura punctulata” OR “Agaricus arvensis” OR “Agelastica alni” OR “Aglia tau” OR “Agrilus anxius” OR “Agriopis aurantiaria” OR “Agriopis marginaria” OR “Agrobacterium radiobacter” OR “Agrochola helvola” OR “Agrotera nemoralis” OR “Agyrium rufum” OR “Alcis jubata” OR “Alcis repandata” OR “Alebra albostriella” OR “Alebra wahlbergi” OR “Alebra wahlbergi” OR “Allantus togatus” OR “Allelochaeta dilophospora” OR “Alnetoidea alneti” OR “Alnetoidia alneti” OR “Alsophila aescularia” OR “Alternaria alternata” OR “Alternaria atra” OR “Alternaria botrytis” OR “Alternaria tenuis” OR “Altica oleracea” OR “Alysidium resinae” OR “Amanita muscaria” OR “Amphipyra pyramidea” OR “Anacampsis blattariella” OR “Anaplectoides prasina” OR “Ancylis tineana” OR “Ancylis uncella” OR “Ancylis upupana” OR “Angerona prunaria” OR “Anisandrus dispar” OR “Anisandrus maiche” OR “Anisogramma virgultorum” OR “Anisostephus betulinus” OR “Annulohypoxylon multiforme” OR “Annulohypoxylon multiforme var. multiforme” OR “Anoplophora chinensis” OR “Anoplophora glabripennis” OR “Anoplus plantaris” OR “Antheraea polyphemus” OR “Apatura ilia” OR “Aphelenchoides fragariae” OR “Aphis fabae” OR “Aphis spiraecola” OR “Apiognomonia errabunda” OR “Apion simile” OR “Apiospora sphaerosperma” OR “Aplosporella alnicola” OR “Aplosporella conglobata” OR “Apocheima hispidaria” OR “Apocheima pilosaria” OR “Apoderus coryli” OR “Apotomis betuletana” OR “Apotomis sororculana” OR “Apotomis turbidana” OR “Apple mosaic virus” OR “Arabis mosaic virus” OR “Arboridia ribauti” OR “Archiearis parthenias” OR “Archips rosana” OR “Arctia caja” OR “Arctornis l‐nigrum” OR “Arge fuscipes” OR “Arge metallica” OR “Arge ustulata” OR “Argyresthia brockeella” OR “Argyresthia glaucinella” OR “Argyresthia goedartella” OR “Argyresthia retinella” OR “Armillaria cepistipes” OR “Armillaria gallica” OR “Armillaria luteobubalina” OR “Armillaria mellea” OR “Armillaria ostoyae” OR “Armillaria tabescens” OR “Arthrinium phaeospermum” OR “Articulospora tetracladia” OR “Aspergillus kanagawaensis” OR “Aspergillus neoniveus” OR “Aspergillus niveus” OR “Aspergillus repens” OR “Aspergillus ruber” OR “Aspergillus versicolor” OR “Asteroma leptothyrioides” OR “Asteroma microspermum” OR “Asterosporium asterospermum” OR “Asterosporium hoffmannii” OR “Asthena albulata” OR “Atemelia torquatella” OR “Athelia epiphylla” OR “Atopospora betulina” OR “Aureobasidium pullulans var. pullulans” OR “Austropaxillus nothofagi” OR “Autographa gamma” OR “Autographa jota” OR “Bactrodesmium betulicola” OR “Bactrodesmium xerophilum” OR “Basidiodendron eyrei” OR “Beauveria bassiana” OR “Bena bicolorana” OR “Berkeleyomyces basicola” OR “Berkleasmium concinnum” OR “Betulaphis brevipilosa” OR “Betulaphis quadrituberculata” OR “Betulina fuscostipitata” OR “Bionectria zelandiaenovae” OR “Bionectria zelandiae‐novae” OR “Birch capillovirus” OR “Birch carlavirus” OR “Birch idaeovirus” OR “Birch leaf roll‐associated virus” OR “Biscogniauxia repanda” OR “Bispora betulina” OR “Bisporella citrina” OR “Biston betularia” OR |
|
“Biston strataria” OR “Bitylenchus maximus” OR “Bjerkandera adusta” OR “Boarmia roboraria” OR “Bohemannia auriciliella” OR “Bohemannia quadrimaculella” OR “Boletus edulis” OR “Boletus scaber” OR “Botryobasidium capitatum” OR “Botryobasidium conspersum” OR “Botryodiplodia conglobata” OR “Botryosphaeria stevensii” OR “Botrytis argillacea” OR “Botrytis cinerea” OR “Brachionycha nubeculosa” OR “Brachysporiella laxa” OR “Brachysporium bloxami” OR “Brachysporium britannicum” OR “Brachysporium fusiforme” OR “Brachysporium nigrum” OR “Brachysporium obovatum” OR “Bryobia rubrioculus” OR “Bucculatrix demaryella” OR “Bulgaria inquinans” OR “Byctiscus betulae” OR “Byctiscus populi” OR “Cabera exanthemata” OR “Cabera pusaria” OR “Cacopsylla affinis” OR “Cactodera betulae” OR “Cacumisporium capitulatum” OR “Cadophora bubakii” OR “Cadophora fastigiata” OR “Cadophora gregata” OR “Caenorhinus mannerheimii” OR “Calaphis betulaecolens” OR “Calaphis betulicola” OR “Calaphis flava” OR “Caliroa annulipes” OR “Caliroa varipes” OR “Callipterinella calliptera” OR “Callipterinella callipterus” OR “Callipterinella minutissima” OR “Callipterinella tuberculata” OR “Calliteara pudibunda” OR “Calocera cornea” OR “Caloptilia betulicola” OR “Caloptilia populetorum” OR “Caloptilia stigmatella” OR “Calosphaeria pulchella” OR “Calosphaeria pusilla” OR “Calosphaeria wahlenbergii” OR “Calycellina dennisii” OR “Calycellina leucella” OR “Calycellina populina” OR “Calycina citrina” OR “Calycina conorum” OR “Camarosporidiella celtidis” OR “Camarosporium betulinum” OR “Campaea margaritata” OR “Camposporium cambrense” OR “Camposporium japonicum” OR “Camposporium pellucidum” OR “Candida albicans” OR “Carpatolechia alburnella” OR “Carpatolechia proximella” OR “Caudospora taleola” OR “Cecidomyia betulae” OR “Cecidophyopsis betulae” OR “Cecidophyopsis vermiformis” OR “Cenococcum geophilum” OR “Ceramica pisi” OR “Ceratocystis catoniana” OR “Ceratocystis piceae” OR “Cercophora caudata” OR “Cerioporus leptocephalus” OR “Cerioporus squamosus” OR “Ceroplastes ceriferus” OR “Cerrena unicolor” OR “Cerrena zonata” OR “Ceuthospora betulae” OR “Chaetochalara bulbosa” OR “Chaetopsis grisea” OR “Chaetosphaeria callimorpha” OR “Chaetosphaeria inaequalis” OR “Chaetosphaeria innumera” OR “Chaetosphaeria myriocarpa” OR “Chaetosphaeria ovoidea” OR “Chaetosphaeria preussii” OR “Chaetosphaeria pulviscula” OR “Chaetosphaeria vermicularioides” OR “Chalara breviclavata” OR “Chalara inflatipes” OR “Cherry leaf roll virus” OR “Chionaspis salicis” OR “Chionodes viduella” OR “Chloridium botryoideum” OR “Chloridium clavaeforme” OR “Chloridium lignicola” OR “Chloridium pachytrachelum” OR “Chloridium preussii” OR “Chloridium virescens var. caudigerum” OR “Chloridium virescens var. chlamydosporum” OR “Chloridium virescens var. virescens” OR “Chlorissa viridata” OR “Chlorociboria aeruginascens” OR “Chlorocillium griseum” OR “Chloroclysta citrata” OR “Chloroclysta miata” OR “Chloroclysta siterata” OR “Chloroclysta truncata” OR “Chondrostereum purpureum” OR “Choreutis diana” OR “Choristoneura diversana” OR “Choristoneura hebenstreitella” OR “Chrysosporium merdarium” OR “Chrysosporium pannorum” OR “Chyliza leptogaster” OR “Ciboria betulae” OR “Cicadetta montana” OR “Cimbex femoratus” OR “Cladosporium cladosporioides” OR “Cladosporium fumago” OR “Cladosporium herbarum” OR “Cladosporium herbarum var. macrocarpum” OR “Cladosporium macrocarpum” OR “Cladosporium nigrellum” OR “Cladosporium sphaerospermum” OR “Claussenomyces atrovirens” OR “Cleora cinctaria” OR “Clethrobius comes” OR “Clonostachys rosea” OR “Clytra quadripunctata” OR “Clytus arietis” OR “Coeliodes rubicundus” OR “Coeliodinus nigritarsis” OR “Coeliodinus rubicundus” OR “Coleophora alnifoliae” OR “Coleophora anatipennella” OR “Coleophora betulella” OR “Coleophora binderella” OR “Coleophora fuscedinella” OR “Coleophora fuscocuprella” OR “Coleophora ibipenella” OR “Coleophora limosipennella” OR “Coleophora milvipennis” OR “Coleophora orbitella” OR “Coleophora potentillae” OR “Coleophora serratella” OR “Coleophora siccifolia” OR “Coleophora violacea” OR “Colletotrichum gloeosporioides” OR “Colocasia coryli” OR “Colotois pennaria” OR “Coltricia focicola” OR “Comstockaspis perniciosa” OR “Coniochaeta ligniaria” OR “Coniochaeta malacotricha” OR “Coniochaeta pulveracea” OR “Coniochaeta subcorticalis” OR “Coniothecium betulinum” OR “Coniothecium complanatum” OR “Coniothecium epidermidis” OR “Coniothyrium fuckelii” OR “Conistra vaccinii” OR “Coprinellus micaceus” OR “Cordana pauciseptata” OR “Coriolus versicolor” OR “Corniculariella urceola” OR “Coronophora angustata” OR “Coronophora gregaria” OR “Coronophora ovipara” OR “Cortinarius paludosaniosus” OR “Cortinarius vernus” OR “Coryne dubia” OR “Corynespora cespitosa” OR “Coryneum betulinum” OR “Coryneum brachyurum” OR “Coryneum disciforme” OR “Coryneum kunzei” OR “Coryneum lanciforme” OR “Cosmia trapezina” OR “Cossus cossus” OR “Crepidodera fulvicornis” OR “Criconema annuliferum” OR “Criconema demani” OR “Criconema princeps” OR “Crocallis elinguaria” OR “Croesus septentrionalis” OR “Crossonema menzeli” OR “Cryptadelphia fusiformis” OR “Cryptocephalus bipunctatus” OR “Cryptocephalus coryli” OR “Cryptocephalus decemmaculatus” OR “Cryptocephalus frontalis” OR “Cryptocephalus labiatus” OR “Cryptocephalus nitidulus” OR “Cryptocephalus parvulus” OR “Cryptocephalus punctiger” OR “Cryptocephalus pusillus” OR “Cryptocephalus sexpunctatus” OR “Cryptocline betularum” OR “Cryptocoryneum condensatum” OR “Cryptorhynchus lapathi” OR “Cryptospora betulae” OR “Cryptosporella betulae” OR “Cryptosporiopsis edgertonii” OR “Cryptosporium betulinum” OR “Cucurbitaria obducens” OR “Cunninghamella elegans” OR “Curculio betulae” OR “Curculio rubidus” OR “Cyclophora albipunctata” OR “Cyclophora linearia” OR “Cyclophora porata” OR “Cyclophora punctaria” OR “Cyclorhipidion pelliculosum” OR “Cylindrocarpon destructans” OR “Cylindrocarpon didymum” OR “Cylindrosporella microsperma” OR “Cylindrosporium betulae” OR “Cylindrotrichum oligospermum” OR “Cyphelium inquinans” OR “Cystopezizella conorum” OR “Cystostereum murrayi” OR “Cystotricha striola” OR “Cytidiella albida” OR “Cytospora ambiens” OR “Cytospora betulina” OR “Cytospora ceratosperma” OR “Cytospora horrida” OR “Cytospora intermedia” OR “Cytospora leucostoma” OR “Cytospora personata” OR “Cytospora populina” OR “Daedalea betulina” OR “Daedalea unicolor” OR “Daedaleopsis confragosa” OR “Daldinia concentrica” OR “Daldinia decipiens” OR “Daldinia lloydii” OR “Daldinia loculata” OR “Daldinia loculatoides” OR “Daldinia vernicosa” OR “Dasineura interbracta” OR “Dasyscyphella nivea” OR “Dasystoma salicella” OR “Deileptenia ribeata” OR “Dematioscypha catenata” OR “Deporaus betulae” OR “Deporaus mannerheimi” OR “Desarmillaria tabescens” OR “Diaporthe alleghaniensis” OR “Diaporthe eres” OR “Diaporthella aristata” OR “Diarsia brunnea” OR “Diarsia dahlii” OR “Diarsia mendica” OR “Diaspidiotus ostreaeformis” OR “Diaspidiotus pyri” OR “Diatrype flavovirens” OR “Diatrype stigma” OR “Diatrype undulata” OR “Diatrypella favacea” OR “Diatrypella melaena” OR “Dicallomera fascelina” OR “Dictyochaeta callimorpha” OR “Didymostilbe eichleriana” OR “Dineura virididorsata” OR “Diplococcium spicatum” OR “Diplodia betulae” OR “Discosia artocreas” OR “Discula betulina” OR “Discula devastans” OR “Disculina betulina” OR “Ditiola peziziformis” OR “Diurnea fagella” OR “Diurnea lipsiella” OR “Dogwood Ringspot Strain of Cherry Leafroll Virus” OR “Dothiora pyrenophora” OR “Dothiorella berengariana f. syringae” OR “Dothiorella pyrenophora” OR “Drepana falcataria” OR “Drepana falcataria falcataria” OR “Drepanothrips reuteri” OR “Drymonia dodonaea” OR “Dysstroma citrata” OR “Dysstroma truncata” OR “Echinosphaeria canescens” OR “Ectoedemia argentipedella” OR “Ectoedemia mediofasciella” OR “Ectoedemia minimella” OR “Ectoedemia occultella” OR “Ectropis bistortata” OR “Ectropis consonaria” OR “Ectropis crepuscularia” OR “Edwardsiana bergmani” OR “Edwardsiana flavescens” OR “Elasmostethus |
|
|
interstinctus” OR “Elasmucha grisea” OR “Electrophaes corylata” OR “Ematurga atomaria” OR “Enargia paleacea” OR “Endomyces vernalis” OR “Endophragmia uniseptata” OR “Endophragmiella fallacia” OR “Endophragmiella oblonga” OR “Endophragmiella suttonii” OR “Endophragmiella tenera” OR “Endophragmiella uniseptata” OR “Endophragmiella uniseptata var. pusilla” OR “Endromis versicolora” OR “Ennomos alniaria” OR “Ennomos autumnaria” OR “Ennomos erosaria” OR “Ennomos quercinaria” OR “Enterobacter cancerogenus” OR “Entomortierella parvispora” OR “Eotetranychus carpini” OR “Eotetranychus coryli” OR “Eotetranychus uncatus” OR “Epicoccum nigrum” OR “Epicoccum purpurascens” OR “Epinotia bilunana” OR “Epinotia brunnichana” OR “Epinotia demarniana” OR “Epinotia immundana” OR “Epinotia ramella” OR “Epinotia solandriana” OR “Epinotia tetraquetrana” OR “Epinotia trigonella” OR “Epione paralellaria” OR “Epirrita autumnata” OR “Epirrita christyi” OR “Epirrita dilutata” OR “Epitrimerus subacromius” OR “Erannis defoliaria” OR “Eriocrania cicatricella” OR “Eriocrania haworthi” OR “Eriocrania salopiella” OR “Eriocrania sangii” OR “Eriocrania semipurpurella” OR “Eriocrania sparrmannella” OR “Eriocrania unimaculella” OR “Eriogaster lanestris” OR “Eriophyes betulinus” OR “Eriophyes leionotus” OR “Eriophyes lissonotus” OR “Eriophyes longisetus” OR “Erisyphe ornata var. europaea” OR “Erysiphe ornata” OR “Erysiphe ornata var. europaea” OR “Euceraphis betulae” OR “Euceraphis punctipennis” OR “Eulecanium ciliatum” OR “Eulecanium douglasi” OR “Eulecanium tiliae” OR “Eulia ministrana” OR “Eulithis testata” OR “Eupithecia satyrata” OR “Euplexia lucipara” OR “Euproctis similis” OR “Eupsilia transversa” OR “Eurhadina concinna” OR “Eurhadina pulchella” OR “Eurois occulta” OR “Eutypa flavovirens” OR “Eutypa hydnoidea” OR “Euura melanocephalus” OR “Euura papillosa” OR “Euura poecilonota” OR “Euura vicina” OR “Euwallacea fornicatus” OR “Euwallacea fornicatus sensu lato” OR “Euwallacea fornicatus sensu stricto” OR “Euwallacea validus” OR “Exaeretia ciniflonella” OR “Excipularia fusispora” OR “Exidia glandulosa” OR “Exidia thuretiana” OR “Exophiala calicioides” OR “Fagocyba cruenta” OR “Falcaria lacertinaria” OR “Femsjonia peziziformis” OR “Fenusa pumila” OR “Fenusa pusilla” OR “Fenusella nana” OR “Fomes annosus” OR “Fomes connatus” OR “Fomes fomentarius” OR “Fomes igniarius” OR “Fomes rufolaccatus” OR “Fomitopsis betulina” OR “Fomitopsis pinicola” OR “Fomitopsis rufolaccata” OR “Furcula bicuspis” OR “Furcula bifida” OR “Fusarium avenaceum” OR “Fusarium lateritium” OR “Fuscoporia laevigata” OR “Fusiccocum betulinum” OR “Fusicladium betulae” OR “Fusicladium scribnerianum” OR “Galerucella lineola” OR “Ganoderma applanatum” OR “Ganoderma australe” OR “Ganoderma lucidum” OR “Ganoderma resinaceum” OR “Gelatinosporium betulinum” OR “Geometra papilionaria” OR “Geotrichum candidum” OR “Gibberella avenacea” OR “Gliomastix murorum var. felina” OR “Gloeosporium betulae” OR “Gloeosporium betulinum” OR “Glomerella cingulata” OR “Gloniopsis praelonga” OR “Glyphina betulae” OR “Glyphina pseudoschrankiana” OR “Glyptotermes brevicornis” OR “Gnomonia betulina” OR “Gnomonia campylostyla” OR “Gnomonia intermedia” OR “Gnomonia setacea” OR “Godronia multispora” OR “Godronia urceolus” OR “Gonatobotrys pallidula” OR “Gonioctena pallida” OR “Gonytrichum caesium var. chloridioides” OR “Gracilia minuta” OR “Graphilbum fragrans” OR “Graphiphora augur” OR “Graphium calicioides” OR “Gymnopus fusipes” OR “Gynaephora selenitica” OR “Halyomorpha halys” OR “Hamamelistes betulinus” OR “Hamamelistes spinosus” OR “Haplographium catenatum” OR “Haplotrichum capitatum” OR “Haplotrichum conspersum” OR “Hebeloma crustuliniforme” OR “Hebeloma leucosarx” OR “Hebeloma velutipes” OR “Hedya atropunctana” OR “Helicogloea exigua” OR “Helicoma dennisii” OR “Helicosporium vegetum” OR “Helicosporium virescens” OR “Heliozela hammoniella” OR “Helminthosporium velutinum” OR “Hemichroa crocea” OR “Hemithea aestivaria” OR “Heringocrania unimaculella” OR “Herminia grisealis” OR “Heterarthrus nemoratus” OR “Heterobasidion annosum” OR “Heterobasidion annosum sensu lato” OR “Heterobasidion parviporum” OR “Heteroborips seriatus” OR “Heterogenea asella” OR “Hormaphis betulae” OR “Hormaphis betulina” OR “Humicola grisea” OR “Hyalophora cecropia” OR “Hyalophora columbia” OR “Hyaloscypha fuscostipitata” OR “Hyaloscypha vitreola” OR “Hydnoporia corrugata” OR “Hydrelia sylvata” OR “Hydriomena impluviata” OR “Hydropisphaera peziza” OR “Hyles gallii” OR “Hylobius abietis” OR “Hymenochaete corrugata” OR “Hymenoscyphus caudatus” OR “Hymenoscyphus tetracladius” OR “Hypatima rhomboidella” OR “Hyphoderma praetermissum” OR “Hypholoma australianum” OR “Hypholoma fasciculare” OR “Hypocrea aureo‐viridis” OR “Hypocrea gelatinosa” OR “Hypocrea rufa” OR “Hypomecis punctinalis” OR “Hypomecis roboraria” OR “Hypomyces corticiicola” OR “Hypotrachyna sorocheila” OR “Hypoxylon howeanum” OR “Hypoxylon multiforme” OR “Hysterium angustatum” OR “Hysterium pulicare” OR “Hysterobrevium curvatum” OR “Idaea aversata” OR “Idaea straminata” OR “Idaea trigeminata” OR “Ileostylus micranthus” OR “Ilyonectria destructans” OR “Immersiella caudata” OR “Incurvaria kivatshella” OR “Incurvaria pectinea” OR “Incurvaria tenuicornis” OR “Inonotus hispidus” OR “Inonotus obliquus” OR “Irpex brevis” OR “Irpex deformis” OR “Irpex hirsutus” OR “Irpex lacteus” OR “Ischnoderma resinosum” OR “Issus coleoptratus” OR “Jackrogersella multiformis” OR “Jodis lactearia” OR “Junghuhnia vincta” OR “Kallistaphis betulicola” OR “Kallistaphis flava” OR “Kretzschmaria deusta” OR “Kybos betulicola” OR “Kybos smaragdula” OR “Lacanobia contigua” OR “Laccaria laccata” OR “Laccaria laccata var. pallidifolia” OR “Laccaria ohiensis” OR “Laccaria tetraspora” OR “Laccaria tetraspora f. major” OR “Lactarius glyciosmus” OR “Lactarius pubescens” OR “Lactarius turpis” OR “Laetiporus sulphureus” OR “Lampronia fuscatella” OR “Lampronia oehlmaniella” OR “Laothoe populi” OR “Laothoë populi” OR “Lasiocampa quercus” OR “Lasiosphaeria canescens” OR “Lasiosphaeria glabrata” OR “Lasiosphaeria hispida” OR “Lasiosphaeria ovina” OR “Lasiosphaeris hispida” OR “Leccinum scabrum” OR “Leccinum schistophilum” OR “Leiopus nebulosus” OR “Lelliottia nimipressuralis” OR “Lentinus brumalis” OR “Lentinus substrictus” OR “Lenzites betulina” OR “Lenzites betulinus” OR “Lepidosaphes conchiformis” OR “Lepidosaphes conchyformis” OR “Lepidosaphes ulmi” OR “Lepidosaphes ussuriensis” OR “Lepista luscina” OR “Leptodontidium elatius var. elatius” OR “Leptographium betulae” OR “Leptographium flavum” OR “Leptographium piriforme” OR “Leptothyrium betulae” OR “Leucodonta bicoloria” OR “Leucoptera malifoliella” OR “Leucostoma auerswaldii” OR “Leucostoma persoonii” OR “Libertella betulina” OR “Libertella favacea” OR “Lindbergina aurovittata” OR “Linnavuoriana decempunctata” OR “Linnemannia gamsii” OR “Linnemannia hyalina” OR “Lithomoia solidaginis” OR “Lithophane socia” OR “Lobesia reliquana” OR “Lochmaea caprea” OR “Lomaspilis marginata” OR “Lomaspilis opis” OR “Lomographa temerata” OR “Lophium arboricola” OR “Luperus flavipes” OR “Luperus longicornis” OR “Lycia hirtaria” OR “Lycia pomonaria” OR “Lycorma delicatula” OR “Lylea tetracoila” OR “Lymantria dispar” OR “Lymantria monacha” OR “Lyonetia clerkella” OR “Lyonetia prunifoliella” OR “Macaria notata” OR “Macrosiphum euphorbiae” OR “Macrothylacia rubi” OR “Macrotyphula juncea” OR “Magdalis carbonaria” OR “Malacosoma neustria” OR “Mamianiella coryli” OR “Marssonia betulae” OR “Marssonina betulae” OR “Massalongia betulifolia” OR “Massalongia rubra” OR “Megachile centuncularis” OR “Melampsoridium betulinum” OR “Melampsoridium hiratsukanum” OR “Melanchra persicariae” OR “Melanchra pisi” OR “Melanconiella decorahensis” OR “Melanconis decorahensis” OR “Melanconis stilbostoma” OR “Melanconium betulinum” OR “Melanconium bicolor” OR “Melanconium parvulum” OR “Melanomma pulvis‐pyrius” OR “Melanomma subdispersum” OR “Melanophila acuminata” OR “Meloidogyne chitwoodi” OR “Meloidogyne fallax” OR “Melolontha melolontha” OR “Memnoniella echinata” OR |
|
|
“Menispora caesia” OR “Menispora ciliata” OR “Menispora glauca” OR “Menispora novogradensis” OR “Menispora tortuosa” OR “Menophra abruptaria” OR “Meripilus giganteus” OR “Merismodes fasciculata” OR “Merulius tremellosus” OR “Messa nana” OR “Metapochonia bulbillosa” OR “Metriostola betulae” OR “Metriotes lutarea” OR “Microsphaera alni” OR “Microsphaera betulae” OR “Microsphaera ornata” OR “Microsphaera ornata var. europaea” OR “Microsphaera ornata var. ornata” OR “Mimas tiliae” OR “Mirandina corticola” OR “Moelleriella betulae” OR “Mollisia albogrisea” OR “Mollisia rosae” OR “Moma alpium” OR “Monaphis antennata” OR “Monodictys castaneae” OR “Monodictys levis” OR “Monodictys paradoxa” OR “Mormo maura” OR “Mortierella gamsii” OR “Mortierella humilis” OR “Mortierella hyalina” OR “Mortierella macrocystis” OR “Mortierella minutissima” OR “Mortierella minutissima var. dubia” OR “Mortierella parvispora” OR “Mortierella verticillata” OR “Mortierella zonata” OR “Mucor moelleri” OR “Mycelium radicis‐atrovirens” OR “Mycosphaerella punctiformis” OR “Myxocyclus polycistis” OR “Myxocyclus polycystis” OR “Myxosporium devastans” OR “Natantiella ligneola” OR “Nectria applanata” OR “Nectria cinnabarina” OR “Nectria cucurbitula” OR “Nectria ditissima” OR “Nectria peziza” OR “Nematinus acuminatus” OR “Nematus latipes” OR “Nematus septentrionalis” OR “Nematus turgaiensis” OR “Nematus umbratus” OR “Nematus viridis” OR “Neofusicoccum australe” OR “Neonectria ditissima” OR “Nepovirus avii” OR “Noctua comes” OR “Noctua fimbriata” OR “Noctua janthina” OR “Nola confusalis” OR “Notodonta dromedaria” OR “Notodonta dromedarius” OR “Nymphalis antiopa” OR “Nymphalis polychloros” OR “Ochropacha duplaris” OR “Ochroporus cinereus” OR “Odontopera bidentata” OR “Odontosia carmelita” OR “Oemona hirta” OR “Oidiodendron cereale” OR “Oidiodendron chlamydosporicum” OR “Oidiodendron echinulatum” OR “Oidiodendron griseum” OR “Oidiodendron tenuissimum” OR “Oligonychus bicolor” OR “Omiodes surrectalis” OR “Oncopsis flavicollis” OR “Oncopsis subangulata” OR “Oncopsis tristis” OR “Oospora cinnabarina” OR “Operophtera brumata” OR “Operophtera fagata” OR “Ophiognomonia intermedia” OR “Ophiognomonia lapponica” OR “Ophiognomonia pseudoischnostyla” OR “Ophiognomonia setacea” OR “Ophiostoma borealis” OR “Ophiostoma canum” OR “Ophiostoma catonianum” OR “Ophiostoma denticiliatum” OR “Ophiostoma floccosum” OR “Ophiostoma karelicum” OR “Ophiostoma pseudokarelicum” OR “Ophiostoma quercus” OR “Ophiostoma sparsiannulatum” OR “Ophiovalsa betulae” OR “Orchestes rusci” OR “Orgyia antiqua” OR “Orgyia recens” OR “Ortholepis betulae” OR “Orthosia cerasi” OR “Orthosia cruda” OR “Orthosia gothica” OR “Orthosia incerta” OR “Orthosia miniosa” OR “Orthosia opima” OR “Orthotaenia undulana” OR “Orthotrichum sainsburyi” OR “Orthotylus marginalis” OR “Otiorhynchus scaber” OR “Otiorhynchus singularis” OR “Ourapteryx sambucaria” OR “Oxyporus populinus” OR “Pachythelia villosella” OR “Pammene obscurana” OR “Pamphilius pallipes” OR “Pamphilius varius” OR “Pandemis cerasana” OR “Pandemis cinnamomeana” OR “Pandemis corylana” OR “Pandemis heperana” OR “Panellus stypticus” OR “Panellus stipticus” OR “Pannaria durietzii” OR “Pannaria leproloma” OR “Panonychus ulmi” OR “Pantilius tunicatus” OR “Papilio canadensis” OR “Pappia fissilis” OR “Paraboeremia putaminum” OR “Parachronistis albiceps” OR “Paradarisa consonaria” OR “Paradarisa extersaria” OR “Paradiarsia sobrina” OR “Parastichtis suspecta” OR “Paratylenchus bukowinensis” OR “Paratylenchus microdorus” OR “Paratylenchus straeleni” OR “Parectropis similaria” OR “Parornix betulae” OR “Parornix loganella” OR “Parthenolecanium corni” OR “Parthenolecanium corni corni” OR “Paxillus cuprinus” OR “Paxillus involutus” OR “Paxillus nothofagi” OR “Pechipogo strigilata” OR “Penicillium adametzii” OR “Penicillium aurantiogriseum” OR “Penicillium brevicompactum” OR “Penicillium brevi‐compactum” OR “Penicillium chrysogenum” OR “Penicillium citreonigrum” OR “Penicillium citrinum” OR “Penicillium commune” OR “Penicillium daleae” OR “Penicillium decumbens” OR “Penicillium dierckxii” OR “Penicillium fellutanum” OR “Penicillium glabrum” OR “Penicillium glaucoalbidum” OR “Penicillium griseoroseum” OR “Penicillium hirsutum” OR “Penicillium janczewskii” OR “Penicillium jensenii” OR “Penicillium lagena” OR “Penicillium lanosum” OR “Penicillium montanense” OR “Penicillium paxilli” OR “Penicillium purpurescens” OR “Penicillium purpurogenum” OR “Penicillium raistrickii” OR “Penicillium simplicissimum” OR “Penicillium solitum” OR “Penicillium solitum var. crustosum” OR “Penicillium spinulosum” OR “Penicillium velutinum” OR “Penicillium waksmanii” OR “Peniophora quercina” OR “Peniophorella praetermissa” OR “Peraxilla tetrapetala” OR “Perenniporia fraxinea” OR “Peribatodes rhomboidaria” OR “Periconia atra” OR “Periconia byssoides” OR “Periconia cambrensis” OR “Periconia cookei” OR “Pezicula cinnamomea” OR “Phacidium betulinum” OR “Phaeomollisia piceae” OR “Phaeotremella foliacea” OR “Phalera bucephala” OR “Phellinus cinereus” OR “Phellinus igniarius” OR “Phellinus laevigatus” OR “Phellinus nigricans” OR “Phenacoccus aceris” OR “Pheosia gnoma” OR “Phialocephala fortinii” OR “Phialophora bubakii” OR “Phialophora cyclaminis” OR “Phialophora fastigiata” OR “Phialophora gregata” OR “Phigalia pilosaria” OR “Phlebia albida” OR “Phlebia tremellosa” OR “Pholiota adiposa” OR “Pholiota subflammans” OR “Phoma corticicola” OR “Phoma putaminum” OR “Phragmotrichum platanoidis” OR “Phratora vulgatissima” OR “Phyllachora betula” OR “Phyllachora betulae‐nanae” OR “Phyllactinia alni” OR “Phyllactinia alnicola” OR “Phyllactinia betulae” OR “Phyllactinia guttata” OR “Phyllactinia suffulta” OR “Phyllobius arborator” OR “Phyllobius argentatus” OR “Phyllobius calcaratus” OR “Phyllobius glaucus” OR “Phyllobius maculicornis” OR “Phyllobius oblongus” OR “Phyllobius pyri” OR “Phyllobius roboretanus” OR “Phyllobius viridicollis” OR “Phyllocoptes lionotus” OR “Phyllonorycter anderidae” OR “Phyllonorycter cavella” OR “Phyllonorycter corylifoliella” OR “Phyllonorycter messaniella” OR “Phyllonorycter ulmifoliella” OR “Phylloporia bistrigella” OR “Phyllosticta betulae” OR “Phytophthora cactorum” OR “Phytophthora cambivora” OR “Phytophthora cinnamomi” OR “Phytophthora cryptogea” OR “Phytophthora gonapodyides” OR “Phytophthora plurivora” OR “Phytophthora pseudosyringae” OR “Phytophthora ramorum” OR “Phytoptus laevis var. lissonotus” OR “Piptoporus betulinus” OR “Plagiodera versicolora” OR “Plagiostoma campylostyla” OR “Plagodis dolabraria” OR “Plagodis pulveraria” OR “Platypus apicalis” OR “Plemeliella betulicola” OR “Plemyria rubiginata” OR “Pleomassaria siparia” OR “Pleotrichocladium opacum” OR “Pleurophragmium rousselianum” OR “Pleurotheciopsis bramleyi” OR “Pleurothecium recurvatum” OR “Pleurotus ostreatus” OR “Pochonia bulbillosa” OR “Podila humilis” OR “Podila minutissima” OR “Podila verticillata” OR “Podofomes mollis” OR “Podosphaera erineophila” OR “Podostictina ardesiaca” OR “Poecilocampa populi” OR “Polia hepatica” OR “Polia nebulosa” OR “Polia trimaculosa” OR “Polydrusus cervinus” OR “Polydrusus flavipes” OR “Polydrusus formosus” OR “Polydrusus marginatus” OR “Polydrusus mollis” OR “Polydrusus pilosus” OR “Polydrusus pterygomalis” OR “Polydrusus tereticollis” OR “Polyporus betulinus” OR “Polyporus brumalis” OR “Polyporus ciliatus” OR “Polyporus leptocephalus” OR “Polyporus melanopus” OR “Polyporus nigricans” OR “Poria obliqua” OR “Praetumpfia obducens” OR “Pratylenchus crenatus” OR “Pratylenchus penetrans” OR “Pristiphora armata” OR “Pristiphora cincta” OR “Pristiphora testacea” OR “Profenusa thomsoni” OR “Prosthemium asterosporum” OR “Prosthemium betulinum” OR “Prosthemium orientale” OR “Protolampra sobrina” OR “Prune dwarf virus” OR “Prunus necrotic ringspot virus” OR “Psallus ambiguus” OR “Psallus perrisi” OR “Pseudocyphellaria granulata” OR “Pseudogymnoascus pannorum” OR “Pseudoinonotus dryadeus” OR “Pseudoips fagana” OR “Pseudoips prasinana” OR “Pseudoips prasinana ssp. Brittanica” OR “Pseudomonas syringae” OR “Pseudomonas syringae pv. syringae” OR |
|
|
“Pseudospiropes longipilus” OR “Pseudospiropes simplex” OR “Pseudotelphusa paripunctella” OR “Pseudovalsa betulae” OR “Pseudovalsa lanciformis” OR “Psylla betulae” OR “Psylla hartigi” OR “Psylliodes picina” OR “Ptilodon capucina” OR “Pulcherricium caeruleum” OR “Pulvinaria betulae” OR “Pulvinaria vitis” OR “Pycnoporus cinnabarinus” OR “Pycnoporus coccineus” OR “Pyrenopeziza betulicola” OR “Pyrenopeziza betulina” OR “Pyrigemmula aurantiaca” OR “Quadraspidiotus ostreaeformis” OR “Ramichloridium anceps” OR “Ramphus pulicarius” OR “Ramularia endophylla” OR “Recurvaria nanella” OR “Resseliella betulicola” OR “Rhamphus pulicarius” OR “Rheumaptera hastata” OR “Rheumaptera hastata ssp. hastata” OR “Rheumaptera hastata ssp. nigrescens” OR “Rheumaptera undulata” OR “Rhinocladiella atrovirens” OR “Rhinotrichella globulifera” OR “Rhizobium radiobacter” OR “Rhizobium rhizogenes” OR “Rhizoctonia solani” OR “Rhizomucor miehei” OR “Rhizopus stolonifer” OR “Rhogogaster punctulata” OR “Rhogogaster scalaris” OR “Rhynchaenus iota” OR “Rhynchaenus rusci” OR “Rhynchaenus stigma” OR “Rhynchaenus testaceus” OR “Rhynchites longiceps” OR “Rhynchites nanus” OR “Ribautiana debilis” OR “Ribautiana tenerrima” OR “Rigidoporus vinctus” OR “Roeslerstammia erxlebella” OR “Russula nitida” OR “Sagenomella diversispora” OR “Saperda populnea” OR “Sarocladium bacillisporum” OR “Saturnia lindia” OR “Saturnia pavonia” OR “Schizophyllum commune” OR “Schizopora paradoxa” OR “Scleroderma bovista” OR “Sclerophoma betulae” OR “Sclerophoma pythiophila” OR “Scolioneura betuleti” OR “Scolioneura vicina” OR “Scolytus intricatus” OR “Scolytus ratzeburgi” OR “Seiridiella ramealis” OR “Selenia dentaria” OR “Selenia lunularia” OR “Selenia tetralunaria” OR “Semioscopis avellanella” OR “Semiothisa carbonaria” OR “Semiothisa notata” OR “Semudobia betulae” OR “Semudobia markakolica” OR “Semudobia skuhravae” OR “Semudobia tarda” OR “Septonema ascedens” OR “Septonema secedens” OR “Septoria betulae” OR “Septoria betulina” OR “Septosporium bulbotrichum” OR “Septotrullula bacilligera” OR “Serraca punctinalis” OR “Sillia ferruginea” OR “Simplicillium lamellicola” OR “Skeletocutis nivea” OR “Solenia confusa” OR “Sorocybe resinae” OR “Spadicoides atra” OR “Spadicoides bina” OR “Spadicoides grovei” OR “Sphaeronema alni” OR “Sphaeropsis alnicola” OR “Sphaerulina betulae” OR “Splanchnonema argus” OR “Splanchnonema siparium” OR “Sporidesmium folliculatum” OR “Sporidesmium tetracoilum” OR “Sporothrix fusiformis” OR “Sporothrix schenckii” OR “Stachybotrys alternans” OR “Stachybotrys echinatus” OR “Stauropus fagi” OR “Stegonosporium muricatum” OR “Steingelia gorodetskia” OR “Stereum hirsutum” OR “Stereum purpureum” OR “Stereum rugosum” OR “Stereum subtomentosum” OR “Sterrhopterix fusca” OR “Sterrhopterix standfussi” OR “Sticta sublimbata” OR “Stigmella betulicola” OR “Stigmella confusella” OR “Stigmella continuella” OR “Stigmella discidia” OR “Stigmella distinguenda” OR “Stigmella lapponica” OR “Stigmella luteella” OR “Stigmella microtheriella” OR “Stigmella sakhalinella” OR “Stigmina quercina” OR “Stomaphis quercus” OR “Strophosoma melanogrammum” OR “Strossmayeria bakeriana” OR “Swammerdamia caesiella” OR “Swammerdamia compunctella” OR “Swammerdamia heroldella” OR “Swammerdamia passerella” OR “Swammerdamia pyrella” OR “Symydobius oblongus” OR “Synanthedon culciformis” OR “Synanthedon culiciformis” OR “Synanthedon scoliaeformis” OR “Synanthedon spheciformis” OR “Synanthedon vespiformis” OR “Syndemis musculana” OR “Syngrapha epigaea” OR “Tachyerges pseudostigma” OR “Tachyerges stigma” OR “Taeniolella exilis” OR “Taeniolina scripta” OR “Tapesia lividofusca” OR “Tapesia rosae” OR “Taphrina betulae” OR “Taphrina betulina” OR “Taphrina carnea” OR “Taphrina nana” OR “Taphrina turgida” OR “Teichospora quercina” OR “Teleiodes paripunctella” OR “Teleiodes wagae” OR “Temnocerus longiceps” OR “Temnocerus nanus” OR “Tetheella fluctuosa” OR “Tetranychus urticae” OR “Tetropium castaneum” OR “Thalera fimbrialis” OR “Thanatephorus cucumeris” OR “Thelonectria applanata” OR “Thrips alni” OR “Thyraylia nana” OR “Thyridium vestitum” OR “Thyronectria coryli” OR “Thysanophora penicillioides” OR “Thysanorea rousseliana” OR “Tobacco necrosis virus” OR “Tomato ringspot virus” OR “Tortricodes alternella” OR “Tortrix viridana” OR “Torula herbarum” OR “Torulomyces lagena” OR “Trametes betulina” OR “Trametes cinnabarina” OR “Trametes coccinea” OR “Trametes gibbosa” OR “Trametes hirsuta” OR “Trametes mollis” OR “Trametes versicolor” OR “Trametes zonata sensu” OR “Trapeliopsis pseudogranulosa” OR “Tremella foliacea” OR “Trichaptum biforme” OR “Trichiosoma lucorum” OR “Trichiura crataegi” OR “Trichocladium asperum” OR “Trichocladium griseum” OR “Trichocladium opacum” OR “Trichoderma aureoviride” OR “Trichoderma koningii” OR “Trichoderma longipilis” OR “Trichoderma polysporum” OR “Trichoderma pseudokoningii” OR “Trichoderma pubescens” OR “Trichoderma strigosum” OR “Trichoderma virens” OR “Trichoderma viride” OR “Trichopteryx carpinata” OR “Trichothecium roseum” OR “Tridelphia heterospora” OR “Trimmatostroma betulinum” OR “Triposporium elegans” OR “Trypodendron domesticum” OR “Trypodendron lineatum” OR “Tubercularia ulmea” OR “Tubercularia vulgaris” OR “Tubeufia cerea” OR “Typhlocyba quercus” OR “Typhula juncea” OR “Typhula ochraceosclerotiata” OR “Tyromyces chioneus” OR “Tyromyces fissilis” OR “Ulocladium botrytis” OR “Ulota viridis” OR “Umbelopsis isabellina” OR “Umbelopsis nana” OR “Umbelopsis ramanniana” OR “Umbelopsis vinacea” OR “Uraba lugens” OR “Valsa ambiens” OR “Valsa auerswaldii” OR “Valsa betulina” OR “Valsa ceratosperma” OR “Valsa leucostoma” OR “Valsella adhaerens” OR “Vanderbylia fraxinea” OR “Varicosporium elodeae” OR “Venturia ditricha” OR “Venusia cambrica” OR “Verticillium dahliae” OR “Verticillium griseum” OR “Vexillomyces atrovirens” OR “Volvaria bombycina” OR “Volvariella bombycina” OR “Watsonalla binaria” OR “Winterella betulae” OR “Xenocriconemella macrodora” OR “Xerocomellus cisalpinus” OR “Xerocomellus ripariellus” OR “Xestia baja” OR “Xestia ditrapezium” OR “Xestia stigmatica” OR “Xestia triangulum” OR “Xiphinema index” OR “Xiphinema rivesi” OR “Xiphydria camelus” OR “Xyleborinus attenuatus” OR “Xyleborinus saxeseni” OR “Xyleborinus saxesenii” OR “Xyleborus dispar” OR “Xyleborus monographus” OR “Xylena solidaginis” OR “Xylococculus betulae” OR “Xylosandrus germanus” OR “Ypsolopha parenthesella” OR “Zalerion arboricola” OR “Zeuzera pyrina” OR “Zygina angusta” OR “Zygorhynchus moelleri” |
In the Table B.2, the search string for B. pubescens used in Web of Science is reported. Totally, 798 papers were retrieved. Titles and abstracts were screened, and 110 pests were added to the list of pests (see Appendix F).
TABLE B.2.
String for Betula pubescens.
| Web of Science All databases |
TOPIC: “Betula pubescens” OR “B. pubescens” OR “Betula alba lusus macrophylla” OR “Betula alba subsp. pubescens” OR “Betula alba f. pubescens” OR “Betula alba var. pubescens” OR “Betula pubescens var. typica” OR “Betula alba” OR “Betula concinna” OR “Betula pubescens subsp. pubescens” OR “common birch” OR “downy birch” OR “swamp birch” OR “white birch” OR “pubescent birch” AND TOPIC: pathogen* OR pathogenic bacteria OR fung* OR oomycet* OR myce* OR bacteri* OR virus* OR viroid* OR insect$ OR mite$ OR phytoplasm* OR arthropod* OR nematod* OR disease$ OR infecti* OR damag* OR symptom* OR pest$ OR vector OR hostplant$ OR “host plant$” OR host OR “root lesion$” OR decline$ OR infestation$ OR damage$ OR symptom$ OR dieback* OR “die back*” OR “malaise” OR aphid$ OR curculio OR thrip$ OR cicad$ OR miner$ OR borer$ OR weevil$ OR “plant bug$” OR spittlebug$ OR moth$ OR mealybug$ OR cutworm$ OR pillbug$ OR “root feeder$” OR caterpillar$ OR “foliar feeder$” OR virosis OR viroses OR blight$ OR wilt$ OR wilted OR canker OR scab$ OR rot OR rots OR rotten OR “damping off” OR “damping‐off” OR blister$ OR “smut” OR mould OR mold OR “damping syndrome$” OR mildew OR scald$ OR “root knot” OR “root‐knot” OR rootknot OR cyst$ OR “dagger” OR “plant parasitic” OR “parasitic plant” OR “plant$parasitic” OR “root feeding” OR “root$feeding” NOT TOPIC: “winged seeds” OR metabolites OR *tannins OR climate OR “maple syrup” OR syrup OR mycorrhiz* OR “carbon loss” OR pollut* OR weather OR propert* OR probes OR spectr* OR antioxidant$ OR transformation OR RNA OR DNA OR “Secondary plant metabolite$” OR metabol* OR “Phenolic compounds” OR Quality OR Abiotic OR Storage OR Pollen* OR fertil* OR Mulching OR Nutrient* OR Pruning OR drought OR “human virus” OR “animal disease*” OR “plant extracts” OR immunological OR “purified fraction” OR “traditional medicine” OR medicine OR mammal* OR bird* OR “human disease*” OR biomarker$ OR “health education” OR bat$ OR “seedling$ survival” OR “anthropogenic disturbance” OR “cold resistance” OR “salt stress” OR salinity OR “aCER method” OR “adaptive cognitive emotion regulation” OR nitrogen OR hygien* OR “cognitive function$” OR fossil$ OR *toxicity OR Miocene OR postglacial OR “weed control” OR landscape NOT TOPIC: “Abraxas sylvata” OR “Acalitus calycophthirus” OR “Acalitus longisetosus” OR “Acalitus longisetus” OR “Acalitus notolius” OR “Acalitus rudis” OR “Acanthosoma haemorrhoidale” OR “Acaphylla acromius” OR “Acarosporium sympodiale” OR “Achlya flavicornis” OR “Acleris emargana” OR “Acleris lipsiana” OR “Acleris logiana” OR “Acleris notana” OR “Acremonium charticola” OR “Acronicta aceris” OR “Acronicta alni” OR “Acronicta americana” OR “Acronicta auricoma” OR “Acronicta dactylina” OR “Acronicta euphorbiae” OR “Acronicta leporina” OR “Acronicta psi” OR “Acronicta rumicis” OR “Aculus leionotus” OR “Adoxophyes orana” OR “Aethalura punctulata” OR “Agaricus arvensis” OR “Agelastica alni” OR “Aglia tau” OR “Agrilus anxius” OR “Agriopis aurantiaria” OR “Agriopis marginaria” OR “Agrobacterium radiobacter” OR “Agrochola helvola” OR “Agromyza alnibetulae” OR “Agrotera nemoralis” OR “Alcis jubata” OR “Alcis repandata” OR “Alebra albostriella” OR “Alebra wahlbergi” OR “Alebra wahlbergi” OR “Allantus togatus” OR “Alnetoidea alneti” OR “Alnetoidia alneti” OR “Alsophila aescularia” OR “Alternaria atra” OR “Altica oleracea” OR “Amphipyra pyramidea” OR “Anacampsis blattariella” OR “Anacampsis populella” OR “Anaplectoides prasina” OR “Ancylis tineana” OR “Ancylis uncella” OR “Ancylis upupana” OR “Angerona prunaria” OR “Anisandrus dispar” OR “Anisogramma virgultorum” OR “Anisostephus betulinus” OR “Anisota senatoria” OR “Annulohypoxylon multiforme” OR “Annulohypoxylon multiforme var. multiforme” OR “Anoplophora chinensis” OR “Anoplus plantaris” OR “Antheraea polyphemus” OR “Aonidomytilus ceanothi” OR “Aphelenchoides fragariae” OR “Apiognomonia errabunda” OR “Apocheima hispidaria” OR “Apocheima pilosaria” OR “Apotomis betuletana” OR “Apotomis sororculana” OR “Apotomis turbidana” OR “Apple mosaic virus” OR “Arabis mosaic virus” OR “Arboridia ribauti” OR “Archiearis parthenias” OR “Archips rosana” OR “Arge fuscipes” OR “Arge ustulata” OR “Argyresthia brockeella” OR “Argyresthia glaucinella” OR “Argyresthia goedartella” OR “Argyresthia retinella” OR “Armillaria cepistipes” OR “Armillaria gallica” OR “Armillaria mellea” OR “Armillaria ostoyae” OR “Armillaria tabescens” OR “Arthopyrenia analepta” OR “Arthopyrenia lapponina” OR “Asteroma leptothyrioides” OR “Asteroma microspermum” OR “Asthena albulata” OR “Atemelia torquatella” OR “Atopospora betulina” OR “Attelabus nitens” OR “Aulacorthum solani” OR “Aureobasidium pullulans var. pullulans” OR “Autographa jota” OR “Automeris io” OR “Bena bicolorana” OR “Betulaphis brevipilosa” OR “Betulaphis quadrituberculata” OR “Betulaphis brevipilosa” OR “Betulaphis quadrituberculata” OR “Betulina fuscostipitata” OR “Birch capillovirus” OR “Birch carlavirus” OR “Birch idaeovirus” OR “Birch leaf roll‐associated virus” OR “Biston betularia” OR “Biston strataria” OR “Bitylenchus maximus” OR “Bjerkandera adusta” OR “Bohemannia auriciliella” OR “Bohemannia quadrimaculella” OR “Botryobasidium pruinatum” OR “Botryosphaeria stevensii” OR “Botrytis argillacea” OR “Botrytis cinerea” OR “Bourdotigloea dura” OR “Brachionycha nubeculosa” OR “Bryobia rubrioculus” OR “Bucculatrix demaryella” OR “Bulgaria inquinans” OR “Byctiscus betulae” OR “Byctiscus populi” OR “Cabera exanthemata” OR “Cabera pusaria” OR “Cacopsylla affinis” OR “Caenorhinus mannerheimii” OR “Calaphis betulicola” OR “Calaphis flava” OR “Caliroa annulipes” OR “Caliroa varipes” OR “Callipterinella calliptera” OR “Callipterinella callipterus” OR “Callipterinella minutissima” OR “Callipterinella tuberculata” OR “Calliteara pudibunda” OR “Caloptilia betulicola” OR “Caloptilia coroniella” OR “Caloptilia populetorum” OR “Caloptilia stigmatella” OR “Calosphaeria pulchella” OR “Calosphaeria wahlenbergii” OR “Calycellina leucella” OR “Calycellina populina” OR “Campaea margaritata” OR “Carpatolechia alburnella” OR “Carpatolechia proximella” OR “Caudospora taleola” OR “Cecidomyia betulae” OR “Cecidophyopsis betulae” OR “Cecidophyopsis vermiformis” OR “Cephaloscypha mairei” OR “Ceramica pisi” OR “Ceratocystis piceae” OR “Ceratomia amyntor” OR “Cerioporus squamosus” OR “Cerrena unicolor” OR “Cheirospora botryospora” OR “Cherry leaf roll virus” OR “Chionaspis furfura” OR “Chionaspis salicis” OR “Chlorissa viridata” OR “Chlorociboria aeruginascens” OR “Chloroclysta miata” OR “Chloroclysta siterata” OR “Chondrostereum purpureum” OR “Choreutis diana” OR “Choristoneura diversana” OR “Choristoneura hebenstreitella” OR “Chrysobothris femorata” OR “Chrysobothris mali” OR “Chrysomela aenea” OR “Chyliza leptogaster” OR “Ciboria betulae” OR “Cicadetta montana” OR “Cimbex femoratus” OR “Cladobotryum mycophilum” OR “Cladosporium macrocarpum” OR “Claussenomyces atrovirens” OR “Cleora cinctaria” OR “Clethrobius comes” OR “Clytra quadripunctata” OR “Clytus arietis” OR “Coeliodinus nigritarsis” OR “Coeliodinus rubicundus” OR “Coleophora alnifoliae” OR “Coleophora anatipennella” OR “Coleophora betulella” OR “Coleophora binderella” OR “Coleophora fuscocuprella” OR “Coleophora milvipennis” OR “Coleophora orbitella” OR “Coleophora potentillae” OR “Coleophora serratella” OR “Coleophora siccifolia” OR “Coleophora violacea” OR “Colocasia coryli” OR “Colotois pennaria” OR “Coltricia focicola” OR “Comstockaspis perniciosa” OR “Conistra vaccinii” OR “Corniculariella urceola” OR |
|
“Coryneum brachyurum” OR “Coryneum disciforme” OR “Coryneum kunzei” OR “Coryneum lanciforme” OR “Coryneum notarisianum” OR “Cosmia trapezina” OR “Cosmospora purtonii” OR “Cosmospora viridescens” OR “Cossus cossus” OR “Crepidodera fulvicornis” OR “Criconema demani” OR “Crocallis elinguaria” OR “Cryptocephalus bipunctatus” OR “Cryptocephalus coryli” OR “Cryptocephalus decemmaculatus” OR “Cryptocephalus nitidulus” OR “Cryptocephalus parvulus” OR “Cryptocephalus punctiger” OR “Cryptocephalus pusillus” OR “Cryptocephalus sexpunctatus” OR “Cryptocline betularum” OR “Cryptorhynchus lapathi” OR “Cryptosporella betulae” OR “Cryptosporium betulinum” OR “Cucurbitaria conglobata” OR “Curculio betulae” OR “Curculio rubidus” OR “Cyclophora albipunctata” OR “Cyclophora linearia” OR “Cyclophora porata” OR “Cyclophora punctaria” OR “Cytospora ambiens” OR “Cytospora betulina” OR “Cytospora coenobitica” OR “Cytospora leucostoma” OR “Cytospora populina” OR “Cytospora tanaitica” OR “Daedalea unicolor” OR “Daedaleopsis confragosa” OR “Daldinia concentrica” OR “Daldinia loculata” OR “Daldinia petriniae” OR “Daldinia vernicosa” OR “Dasineura fastidiosa” OR “Dasineura interbracta” OR “Dasystoma salicella” OR “Datana ministra” OR “Deileptenia ribeata” OR “Deporaus betulae” OR “Desarmillaria tabescens” OR “Diaporthe eres” OR “Diarsia brunnea” OR “Diarsia dahlii” OR “Diarsia mendica” OR “Diaspidiotus lenticularis” OR “Diaspidiotus ostreaeformis” OR “Diaspidiotus pyri” OR “Diatrype disciformis” OR “Diatrype stigma” OR “Diatrype undulata” OR “Diatrypella decorata” OR “Diatrypella favacea” OR “Dicallomera fascelina” OR “Didymostilbe eichleriana” OR “Dineura virididorsata” OR “Diplosis betulicola” OR “Diplosis betulina” OR “Discosia artocreas” OR “Discula betulina” OR “Disculina betulina” OR “Diurnea fagella” OR “Diurnea lipsiella” OR “Dogwood Ringspot Strain of Cherry Leafroll Virus” OR “Dothidella betulina” OR “Drepana arcuata” OR “Drepana bilineata” OR “Drepana falcataria” OR “Drepana falcataria falcataria” OR “Drepanothrips reuteri” OR “Dysstroma citrata” OR “Dysstroma truncata” OR “Eacles imperialis” OR “Ectoedemia minimella” OR “Ectoedemia occultella” OR “Ectropis bistortata” OR “Ectropis crepuscularia” OR “Edwardsiana bergmani” OR “Edwardsiana flavescens” OR “Elasmostethus interstinctus” OR “Elasmucha grisea” OR “Electrophaes corylata” OR “Ematurga atomaria” OR “Enargia paleacea” OR “Endromis versicolora” OR “Ennomos alniaria” OR “Ennomos autumnaria” OR “Ennomos erosaria” OR “Ennomos quercinaria” OR “Enterobacter cancerogenus” OR “Eotetranychus carpini” OR “Eotetranychus querci” OR “Eotetranychus uncatus” OR “Epicoccum nigrum” OR “Epinotia bilunana” OR “Epinotia brunnichana” OR “Epinotia demarniana” OR “Epinotia immundana” OR “Epinotia ramella” OR “Epinotia solandriana” OR “Epinotia tetraquetrana” OR “Epinotia trigonella” OR “Epirrita autumnata” OR “Epirrita christyi” OR “Epirrita dilutata” OR “Epitrimerus subacromius” OR “Erannis defoliaria” OR “Eriocrania cicatricella” OR “Eriocrania haworthi” OR “Eriocrania salopiella” OR “Eriocrania sangii” OR “Eriocrania semipurpurella” OR “Eriocrania sparrmannella” OR “Eriocrania unimaculella” OR “Eriogaster lanestris” OR “Eriophyes leionotus” OR “Eriophyes lissonotus” OR “Erisyphe ornata var. europaea” OR “Erysiphe ornata” OR “Erysiphe ornata var. europaea” OR “Erysiphe ornata var. ornata” OR “Euceraphis betulae” OR “Euceraphis punctipennis” OR “Euceraphis betulae” OR “Euceraphis punctipennis” OR “Eulecanium ciliatum” OR “Eulecanium douglasi” OR “Eulecanium tiliae” OR “Eulecanium transvittatum” OR “Eulia ministrana” OR “Eulithis testata” OR “Eupithecia satyrata” OR “Euplexia lucipara” OR “Euproctis similis” OR “Eupsilia transversa” OR “Eurhadina concinna” OR “Eurhadina pulchella” OR “Eurois occulta” OR “Eutypa aterrima” OR “Euura melanocephalus” OR “Euura papillosa” OR “Euura poecilonota” OR “Euura vicina” OR “Exaeretia ciniflonella” OR “Exidia repanda” OR “Exosporium disciforme” OR “Fagocyba cruenta” OR “Falcaria lacertinaria” OR “Fenestella betulae” OR “Fenusa pumila” OR “Fenusa pusilla” OR “Fenusella nana” OR “Fomes annosus” OR “Fomes applanatus” OR “Fomes connatus” OR “Fomes fomentarius” OR “Fomes igniarius” OR “Fomes igniarius var. laevigatus” OR “Fomitopsis betulina” OR “Fomitopsis pinicola” OR “Furcula bicuspis” OR “Furcula bifida” OR “Fusarium avenaceum” OR “Fusarium lateritium” OR “Fusicladium betulae” OR “Fusicoccum betulae” OR “Ganoderma applanatum” OR “Ganoderma australe” OR “Ganoderma lucidum” OR “Ganoderma resinaceum” OR “Geometra papilionaria” OR “Gloeosporium betulae” OR “Gloeosporium betulinum” OR “Glyphina betulae” OR “Glyphina pseudoschrankiana” OR “Glyphina schrankiana” OR “Gnomonia betulae‐pubescentis” OR “Gnomonia setacea” OR “Godronia urceolus” OR “Gonatobotrys pallidula” OR “Gonioctena pallida” OR “Gracilia minuta” OR “Graphiphora augur” OR “Gymnopus fusipes” OR “Gynaephora selenitica” OR “Halysidota tessellaris” OR “Hamamelistes betulinus” OR “Hedya atropunctana” OR “Helicogloea septifera” OR “Heliococcus osborni” OR “Heliozela hammoniella” OR “Hemichroa crocea” OR “Hemithea aestivaria” OR “Heringocrania unimaculella” OR “Herminia grisealis” OR “Heterarthrus nemoratus” OR “Heterobasidion annosum” OR “Heterobasidion annosum sensu lato” OR “Heterogenea asella” OR “Hormaphis betulae” OR “Hormomyia rubra” OR “Hyalophora cecropia” OR “Hyaloscypha fuscostipitata” OR “Hyaloscypha vitreola” OR “Hyaloscypha vraolstadiae” OR “Hydrelia sylvata” OR “Hydriomena impluviata” OR “Hypatima rhomboidella” OR “Hyphantria cunea” OR “Hyphoderma setigerum” OR “Hypholoma fasciculare” OR “Hypocrea strictipilosa” OR “Hypomecis punctinalis” OR “Hypomecis roboraria” OR “Hypomecis umbrosaria” OR “Hypoxylon fuscum” OR “Hypoxylon multiforme” OR “Hysterium pulicare” OR “Hysterobrevium curvatum” OR “Hysterographium flexuosum” OR “Idaea aversata” OR “Idaea straminata” OR “Idaea trigeminata” OR “Immotthia atrograna” OR “Immotthia hypoxylon” OR “Incurvaria pectinea” OR “Inonotus hispidus” OR “Inonotus obliquus” OR “Irpex cremicolor” OR “Issus coleoptratus” OR “Jackrogersella multiformis” OR “Jodis lactearia” OR “Kallistaphis betulicola” OR “Kallistaphis flava” OR “Kretzschmaria deusta” OR “Kybos betulicola” OR “Kybos smaragdula” OR “Lacanobia contigua” OR “Laetiporus sulphureus” OR “Lampronia fuscatella” OR “Laothoe populi” OR “Lasiosphaeria ovina” OR “Leiopus nebulosus” OR “Lelliottia nimipressuralis” OR “Lentinus brumalis” OR “Lentinus substrictus” OR “Lenzites betulinus” OR “Lepidosaphes conchiformis” OR “Lepidosaphes ulmi” OR “Leucoptera malifoliella” OR “Leucoptera scitella” OR “Lindbergina aurovittata” OR “Linnavuoriana decempunctata” OR “Lithophane hepatica” OR “Lithophane socia” OR “Lobesia reliquana” OR “Lobophora halterata” OR “Lochmaea caprea” OR “Lophocampa caryae” OR “Luperus flavipes” OR “Luperus longicornis” OR “Lycia hirtaria” OR “Lygocoris pabulinus” OR “Lymantria dispar” OR “Lymantria monacha” OR “Lyonetia clerkella” OR “Lyonetia prunifoliella” OR “Macaria notata” OR “Malacosoma americana” OR “Malacosoma neustria” OR “Mamianiella coryli” OR “Marssonina betulae” OR “Massalongia betulifolia” OR “Massalongia rubra” OR “Megachile centuncularis” OR “Melampsora betulina” OR “Melampsoridium betulae” OR “Melampsoridium betulinum” OR “Melanchra persicariae” OR “Melanchra pisi” OR “Melanconis alni” OR “Melanconis stilbostoma” OR “Melanconium betulinum” OR “Melanconium bicolor” OR “Melanconium zonatum” OR “Melanomma pulvis‐pyrius” OR “Melanophila acuminata” OR “Meliniomyces vraolstadiae” OR “Meloidogyne chitwoodi” OR “Melolontha melolontha” OR “Menophra abruptaria” OR “Meripilus giganteus” OR “Messa nana” OR “Metriostola betulae” OR “Microsphaera alni” OR “Microsphaera betulae” OR “Microsphaera ornata” OR “Microsphaera ornata var. europaea” OR “Microsphaera ornata var. ornata” OR “Mimas tiliae” OR “Mollisia cinerea” OR “Mollisia rosae” OR “Moma alpium” OR “Monaphis antennata” OR “Mormo maura” OR “Mycosphaerella punctiformis” OR “Nectria cinnabarina” OR “Nectria coccinea” OR “Nectria ditissima” OR “Nectria flava” OR “Nectria galligena” OR “Nectria purtoni” OR “Nectria viridescens” OR “Nematinus acuminatus” OR “Nematus latipes” OR “Nematus septentrionalis” OR “Nematus umbratus” |
|
|
OR “Neofusicoccum australe” OR “Neonectria coccinea” OR “Neonectria ditissima” OR “Noctua comes” OR “Noctua fimbriata” OR “Noctua janthina” OR “Nola confusalis” OR “Notodonta dromedarius” OR “Nymphalis antiopa” OR “Nymphalis vau‐album” OR “Ochropacha duplaris” OR “Ochroporus cinereus” OR “Odontopera bidentata” OR “Odontosia carmelita” OR “Olethreutes zelleriana” OR “Oligocentria lignicolor” OR “Oncopsis flavicollis” OR “Oncopsis subangulata” OR “Oncopsis tristis” OR “Oospora cinnabarina” OR “Operophtera brumata” OR “Operophtera fagata” OR “Ophiognomonia intermedia” OR “Ophiognomonia ischnostyla” OR “Ophiognomonia lapponica” OR “Ophiognomonia pseudoischnostyla” OR “Ophiognomonia setacea” OR “Ophiostoma borealis” OR “Ophiostoma denticiliatum” OR “Ophiostoma karelicum” OR “Ophiostoma quercus” OR “Ophiovalsa betulae” OR “Opisthograptis luteolata” OR “Orchestes rusci” OR “Orgyia antiqua” OR “Orgyia leucostigma” OR “Orgyia recens” OR “Ortholepis betulae” OR “Orthosia cerasi” OR “Orthosia cruda” OR “Orthosia gothica” OR “Orthosia incerta” OR “Orthosia miniosa” OR “Orthosia opima” OR “Orthotaenia undulana” OR “Orthotylus marginalis” OR “Otiorhynchus scaber” OR “Otiorhynchus singularis” OR “Ourapteryx sambucaria” OR “Oxyporus populinus” OR “Paleacrita vernata” OR “Pammene obscurana” OR “Pamphilius pallipes” OR “Pamphilius varius” OR “Pandemis cerasana” OR “Pandemis cinnamomeana” OR “Pandemis corylana” OR “Pandemis heparana” OR “Pandemis heperana” OR “Panonychus ulmi” OR “Pantilius tunicatus” OR “Papilio glaucus” OR “Pappia fissilis” OR “Parachronistis albiceps” OR “Paradarisa consonaria” OR “Paranthrene tabaniformis” OR “Parastichtis suspecta” OR “Paratylenchus bukowinensis” OR “Paratylenchus microdorus” OR “Paratylenchus straeleni” OR “Parectropis similaria” OR “Parornix betulae” OR “Parornix loganella” OR “Parthenolecanium corni” OR “Pechipogo strigilata” OR “Pellicularia pruinata” OR “Peniophora cinerea” OR “Peniophora quercina” OR “Peniophora setigera” OR “Perenniporia fraxinea” OR “Peribatodes rhomboidaria” OR “Phalera bucephala” OR “Phellinus cinereus” OR “Phellinus igniarius” OR “Phellinus laevigatus” OR “Phenacoccus aceris” OR “Pheosia gnoma” OR “Phialophora verrucosa” OR “Phigalia pilosaria” OR “Phobetron pithecium” OR “Phratora vulgatissima” OR “Phyllactinia alnicola” OR “Phyllactinia betulae” OR “Phyllactinia corylea” OR “Phyllactinia guttata” OR “Phyllactinia suffulta” OR “Phyllobius argentatus” OR “Phyllobius glaucus” OR “Phyllobius maculicornis” OR “Phyllobius oblongus” OR “Phyllobius pyri” OR “Phyllobius roboretanus” OR “Phyllobius viridicollis” OR “Phyllocoptes lionotus” OR “Phyllonorycter anderidae” OR “Phyllonorycter cavella” OR “Phyllonorycter corylifoliella” OR “Phyllonorycter messaniella” OR “Phyllonorycter ulmifoliella” OR “Phylloporia bistrigella” OR “Phyllosticta betulae” OR “Phytobia betulae” OR “Phytophthora cactorum” OR “Phytophthora cambivora” OR “Phytophthora gonapodyides” OR “Phytophthora plurivora” OR “Phytophthora pseudosyringae” OR “Phytophthora ramorum” OR “Piptoporus betulinus” OR “Plagiodera versicolora” OR “Plagodis dolabraria” OR “Plagodis pulveraria” OR “Plemeliella betulicola” OR “Plemyria rubiginata” OR “Pleomassaria siparia” OR “Pleurotus ostreatus” OR “Plowrightia virgultorum” OR “Poecilocampa populi” OR “Polia hepatica” OR “Polia nebulosa” OR “Polydrusus cervinus” OR “Polydrusus flavipes” OR “Polydrusus formosus” OR “Polydrusus marginatus” OR “Polydrusus mollis” OR “Polydrusus pilosus” OR “Polydrusus pterygomalis” OR “Polydrusus tereticollis” OR “Polygonia c‐album” OR “Polyporus betulinus” OR “Polyporus brumalis” OR “Polyporus ciliatus” OR “Polyporus melanopus” OR “Polyporus zonatus” OR “Poria obliqua” OR “Pratylenchus penetrans” OR “Pristiphora armata” OR “Pristiphora cincta” OR “Pristiphora testacea” OR “Profenusa thomsoni” OR “Prosthemium asterosporum” OR “Protolampra sobrina” OR “Prune dwarf virus” OR “Prunus necrotic ringspot virus” OR “Psallus ambiguus” OR “Psallus perrisi” OR “Pseudodiplodia ligniaria” OR “Pseudoinonotus dryadeus” OR “Pseudoips prasinana” OR “Pseudoips prasinana ssp. Brittanica” OR “Pseudomonas syringae pv. syringae” OR “Pseudotelphusa paripunctella” OR “Pseudovalsa lanciformis” OR “Psyche crassiorella” OR “Psyche rotunda” OR “Psylla betulae” OR “Psylla hartigi” OR “Psylliodes picina” OR “Ptilodon capucina” OR “Pulvinaria vitis” OR “Pycnopeziza sympodialis” OR “Pyrenopeziza betulicola” OR “Pyrenopeziza betulina” OR “Pyrrharctia isabella” OR “Radulum radula” OR “Ramphus pulicarius” OR “Ramularia endophylla” OR “Recurvaria nanella” OR “Resseliella betulicola” OR “Rhamphus pulicarius” OR “Rheumaptera hastata” OR “Rheumaptera hastata ssp. hastata” OR “Rheumaptera hastata ssp. nigrescens” OR “Rheumaptera subhastata” OR “Rheumaptera undulata” OR “Rhizobium rhizogenes” OR “Rhizoctonia solani” OR “Rhogogaster punctulata” OR “Rhogogaster scalaris” OR “Rhynchaenus iota” OR “Rhynchaenus rusci” OR “Ribautiana debilis” OR “Ribautiana tenerrima” OR “Roeslerstammia erxlebella” OR “Rutstroemia bolaris” OR “Saperda populnea” OR “Saturnia pavonia” OR “Schizophyllum commune” OR “Schizura concinna” OR “Schizura unicornis” OR “Scolioneura betuleti” OR “Scolioneura vicina” OR “Scolytus intricatus” OR “Scolytus ratzeburgi” OR “Selenia dentaria” OR “Selenia lunularia” OR “Selenia tetralunaria” OR “Semioscopis avellanella” OR “Semiothisa notata” OR “Semudobia betulae” OR “Semudobia skuhravae” OR “Semudobia tarda” OR “Septoria betulae” OR “Septoria betulae‐odoratae” OR “Septoria betulicola” OR “Septoria betulina” OR “Sphaeronema alni” OR “Sphaeropsis betulae” OR “Sphaeropsis betulae var. macrospora” OR “Sphaerulina betulae” OR “Splanchnonema argus” OR “Stauropus fagi” OR “Stereum purpureum” OR “Stereum rugosum” OR “Sterrhopterix standfussi” OR “Stigmella betulicola” OR “Stigmella bistrimaculella” OR “Stigmella confusella” OR “Stigmella continuella” OR “Stigmella discidia” OR “Stigmella lapponica” OR “Stigmella luteella” OR “Stigmella occultella” OR “Stigmella sakhalinella” OR “Stigmina pulvinata” OR “Stomaphis quercus” OR “Strophosoma melanogrammum” OR “Stylonectria purtonii” OR “Swammerdamia caesiella” OR “Swammerdamia compunctella” OR “Swammerdamia passerella” OR “Swammerdamia pyrella” OR “Symydobius oblongus” OR “Synanthedon culciformis” OR “Synanthedon culiciformis” OR “Synanthedon scoliaeformis” OR “Synanthedon spheciformis” OR “Synanthedon vespiformis” OR “Syndemis musculana” OR “Syngrapha parilis” OR “Tachyerges pseudostigma” OR “Tachyerges stigma” OR “Taeniolella exilis” OR “Taeniolina scripta” OR “Tapesia rosae” OR “Taphrina alpina” OR “Taphrina bacteriosperma” OR “Taphrina betulae” OR “Taphrina betulina” OR “Taphrina carnea” OR “Taphrina lapponica” OR “Taphrina nana” OR “Taphrina splendens” OR “Teleiodes wagae” OR “Temnocerus longiceps” OR “Temnocerus nanus” OR “Tetheella fluctuosa” OR “Tetranychus turkestani” OR “Tetranychus urticae” OR “Thanatephorus cucumeris” OR “Thrips alni” OR “Thyraylia nana” OR “Thyronectria coryli” OR “Tobacco necrosis virus” OR “Tomato ringspot virus” OR “Tortricodes alternella” OR “Tortrix viridana” OR “Trametes betulina” OR “Trametes hirsuta” OR “Trametes versicolor” OR “Tremex fuscicornis” OR “Trichiosoma lucorum” OR “Trichiura crataegi” OR “Trichoderma strictipile” OR “Trichopteryx carpinata” OR “Trimmatostroma betulinum” OR “Trypodendron domesticum” OR “Tubercularia vulgaris” OR “Tubeufia cerea” OR “Typhlocyba quercus” OR “Tyromyces chioneus” OR “Tyromyces fissilis” OR “Uncinula betulae” OR “Valdensia heterodoxa” OR “Valdensinia heterodoxa” OR “Valsa coenobitica” OR “Valsa leucostoma” OR “Vanderbylia fraxinea” OR “Venturia ditricha” OR “Venturia glacialis” OR “Venusia cambrica” OR “Vexillomyces atrovirens” OR “Watsonalla binaria” OR “Winterella betulae” OR “Xenocriconemella macrodora” OR “Xenotypa aterrima” OR “Xestia baja” OR “Xestia ditrapezium” OR “Xestia stigmatica” OR “Xestia triangulum” OR “Xiphinema index” OR “Xiphinema rivesi” OR “Xiphydria camelus” OR “Xylaria polymorpha” OR “Xyleborinus saxeseni” OR “Xyleborinus saxesenii” OR “Xyleborus dispar” OR “Xyleborus monographus” OR “Xylena solidaginis” OR “Xylococculus betulae” OR “Xylodon radula” OR “Xylosandrus germanus” OR “Ypsolopha parenthesella” OR “Zeuzera pyrina” OR “Zygina angusta” |
APPENDIX C. Plant taxa reported to be present in the nurseries of Betula pendula and B. pubescens
C.1.
TABLE C.1 Plant taxa reported in the Dossier Sections 3.1 and 3.2 to be present in the nurseries of B. pendula and B. pubescens.
| Number | Plant taxa | Number | Plant taxa |
|---|---|---|---|
| 1 | Abelia | 703 | Malus ‘Rosehip’ |
| 2 | Abies alba | 704 | Malus ‘Rosemary Russet’ |
| 3 | Abies concolor | 705 | Malus ‘Rosette’ |
| 4 | Abies concolor ‘Violacea’ | 706 | Malus ‘Royal Beauty’ |
| 5 | Abies fraseri | 707 | Malus ‘Royalty’ |
| 6 | Abies grandis | 708 | Malus ‘Rudolph’ |
| 7 | Abies koreana | 709 | Malus ‘Santana’ |
| 8 | Abies nobilis | 710 | Malus ‘Saturn’ |
| 9 | Abies nordmanniana | 711 | Malus ‘Scarlet Brandywine’ |
| 10 | Abies procera | 712 | Malus ‘Scarlett’ |
| 11 | Acacia | 713 | Malus ‘Scotch Bridget’ |
| 12 | Acanthus | 714 | Malus ‘Scotch Dumpling’ |
| 13 | Acer | 715 | Malus ‘Scrumptious’ |
| 14 | Acer campestre | 716 | Malus ‘Somerset Redstreak’ |
| 15 | Acer campestre ‘Elsrijk’ | 717 | Malus ‘Spartan’ |
| 16 | Acer campestre fastigiata | 718 | Malus ‘St Edmund's Russet’ |
| 17 | Acer campestre ‘Streetwise’ | 719 | Malus ‘Stirling Castle’ |
| 18 | Acer campestre ‘William Caldwell’ | 720 | Malus ‘Stoke Red’ |
| 19 | Acer capillipes | 721 | Malus Sun Rival |
| 20 | Acer cappadocicum ‘Aureum’ | 722 | Malus ‘Sunset’ |
| 21 | Acer cappadocicum ‘Rubrum’ | 723 | Malus ‘Surprize’ |
| 22 | Acer davidii | 724 | Malus sylvestris |
| 23 | Acer davidii ‘George Forrest’ | 725 | Malus ‘Three Counties’ |
| 24 | Acer davidii ‘Viper’ | 726 | Malus ‘Tickled Pink Baya Marisa’ |
| 25 | Acer ‘Esk Flamingo’ | 727 | Malus ‘Tom Putt’ |
| 26 | Acer griseum | 728 | Malus toringo subsp. sargentii ‘Tina’ |
| 27 | Acer lobelii | 729 | Malus transitoria |
| 28 | Acer macrocarpa | 730 | Malus transitoria ‘Thornhayes Tansy’ |
| 29 | Acer negundo ‘Flamingo’ | 731 | Malus ‘Tremlett's Bitter’ |
| 30 | Acer negundo ‘Kelly's Gold’ | 732 | Malus trilobata |
| 31 | Acer negundo ‘Winter Lightning’ | 733 | Malus trilobata ‘Guardsman’ |
| 32 | Acer orientalia | 734 | Malus ‘Trinity’ |
| 33 | Acer palmatum | 735 | Malus tschonoskii |
| 34 | Acer palmatum ‘Atropurpureum’ | 736 | Malus tschonoskii ‘Belmonte’ |
| 35 | Acer palmatum ‘Crimson Queen’ | 737 | Malus ‘Van Eseltine’ |
| 36 | Acer palmatum ‘Dissectum’ | 738 | Malus ‘Vicky’ |
| 37 | Acer palmatum ‘Enkan’ | 739 | Malus ‘Warner's King’ |
| 38 | Acer palmatum ‘Garnet’ | 740 | Malus ‘William Crump’ |
| 39 | Acer palmatum ‘Katsura’ | 741 | Malus ‘Winter Gem’ |
| 40 | Acer palmatum ‘Kinshi’ | 742 | Malus ‘Worcester Pearmain’ |
| 41 | Acer palmatum ‘Linearilobum’ | 743 | Malus × moerlandsii ‘Profusion Improved’ |
| 42 | Acer palmatum ‘Orange Dream’ | 744 | Malus × robusta ‘Red Sentinel’ |
| 43 | Acer palmatum ‘Osakazuki’ | 745 | Malus ‘Yarlington Mill’ |
| 44 | Acer palmatum ‘Pixie’ | 746 | Matteuccia |
| 45 | Acer palmatum ‘Red Wings’ | 747 | Maytenus boaria |
| 46 | Acer palmatum ‘Sango kaku’ | 748 | Meconopsis |
| 47 | Acer palmatum ‘Seiryu’ | 749 | Mespilus ‘Nottingham’ |
| 48 | Acer palmatum ‘Shaina’ | 750 | Metasequoia glyptostroboides |
| 49 | Acer palmatum ‘Suminagashi’ | 751 | Miscanthus |
| 50 | Acer palmatum ‘Tamukeyama’ | 752 | Molinia |
| 51 | Acer palmatum ‘Trompenburg’ | 753 | Monarda |
| 52 | Acer palmatum ‘Villa Taranto’ | 754 | Morus ‘Carman’ |
| 53 | Acer pensylvanicum | 755 | Morus ‘Chelsea’ |
| 54 | Acer platanoides | 756 | Morus ‘Giant Fruit’ |
| 55 | Acer platanoides ‘Columnare’ | 757 | Morus ‘Mojo Berry’ |
| 56 | Acer platanoides ‘Crimson King’ | 758 | Morus ‘Pendula’ |
| 57 | Acer platanoides ‘Crimson Sentry’ | 759 | Myrtus |
| 58 | Acer platanoides ‘Deborah’ | 760 | Nandina |
| 59 | Acer platanoides ‘Drummondii’ | 761 | Nemesia |
| 60 | Acer platanoides ‘Emerald Queen’ | 762 | Nepeta |
| 61 | Acer platanoides ‘Globosum’ | 763 | Nothofagus |
| 62 | Acer platanoides ‘Perfect Upright’ | 764 | Nothofagus antarctica |
| 63 | Acer platanoides ‘Princeton Gold’ | 765 | Nyssa sylvatica |
| 64 | Acer pseudoplatanus | 766 | Nyssa sylvatica ‘Red Rage’ |
| 65 | Acer pseudoplatanus ‘Brilliantissimum’ | 767 | Nyssa sylvatica ‘Wisley Bonfire’ |
| 66 | Acer pseudoplatanus ‘Erectum’ | 768 | Olea europea |
| 67 | Acer pseudoplatanus ‘Esk Sunset’ | 769 | Olearia |
| 68 | Acer pseudoplatanus ‘Leopoldii’ | 770 | Ophiopogon |
| 69 | Acer pseudoplatanus ‘Prinz Handjery’ | 771 | Osmanthus |
| 70 | Acer pseudoplatanus purpurea | 772 | Osmunda |
| 71 | Acer rubrum | 773 | Ostrya carpinifolia |
| 72 | Acer rubrum ‘Autumn Flame’ | 774 | Pachysandra |
| 73 | Acer rubrum ‘Brandywine’ | 775 | Pachystegia |
| 74 | Acer rubrum ‘Karpick’ | 776 | Paeonia |
| 75 | Acer rubrum ‘October Glory’ | 777 | Panicum |
| 76 | Acer rubrum ‘Red Sunset’ | 778 | Parrotia persica |
| 77 | Acer rubrum ‘Scanlon’ | 779 | Parrotia persica ‘Bella’ |
| 78 | Acer rubrum ‘Sun Valley’ | 780 | Parrotia persica ‘Persian Spire’ |
| 79 | Acer saccharum | 781 | Parrotia persica ‘Vanessa’ |
| 80 | Acer shirasawanum ‘Autumn Moon’ | 782 | Paulownia tomentosa |
| 81 | Acer tataricum subsp. ginnala | 783 | Pennisetum |
| 82 | Acer × freemanii ‘Armstrong’ | 784 | Penstemon |
| 83 | Acer × freemanii ‘Autumn Blaze’ | 785 | Perovskia |
| 84 | Acer × freemanii ‘Morgan’ | 786 | Persicaria |
| 85 | Achillea | 787 | Philadelphus |
| 86 | Acorus | 788 | Phlomis |
| 87 | Actaea | 789 | Phlox |
| 88 | Aesculus hippocastanum ‘Baumannii’ | 790 | Phormium |
| 89 | Aesculus indica | 791 | Photinia |
| 90 | Aesculus parviflora | 792 | Photinia × fraseri ‘Red Robin’ |
| 91 | Aesculus × carnea ‘Briotii’ | 793 | Phygelius |
| 92 | Agapanthus | 794 | Physocarpus |
| 93 | Agastache | 795 | Physocarpus opulifolius ‘Diablo’ |
| 94 | Ajuga | 796 | Physocarpus opulifolius ‘Lady in Red’ |
| 95 | Akebia | 797 | Physostegia |
| 96 | Albizia julibrissin ‘Chocolate Fountain’ | 798 | Picea abies |
| 97 | Albizia julibrissin ‘Evys Pride’ | 799 | Picea omorika |
| 98 | Albizia julibrissin ‘Ombrella’ | 800 | Picea orientalis |
| 99 | Albizia julibrissin ‘Shidare’ | 801 | Picea ormorika |
| 100 | Albizia julibrissin ‘Summer Chocolate’ | 802 | Picea pungens ‘Erich Frahm’ |
| 101 | Alchemilla | 803 | Picea pungens glauca |
| 102 | Allium | 804 | Picea pungens ‘Iseli Fastigiate’ |
| 103 | Alnus | 805 | Picea sitchensis |
| 104 | Alnus cordata | 806 | Picea smithiana ‘Aurea’ |
| 105 | Alnus glutinosa | 807 | Pinus |
| 106 | Alnus glutinosa ‘Imperialis’ | 808 | Pinus densiflora ‘Umbraculifera’ |
| 107 | Alnus glutinosa ‘Laciniata’ | 809 | Pinus flexilis ‘Vanderwolf's Pyramid’ |
| 108 | Alnus incana | 810 | Pinus mugo ‘Winter Sun’ |
| 109 | Alnus incana ‘Aurea’ | 811 | Pinus nigra |
| 110 | Alnus rubra | 812 | Pinus nigra ‘Bright Eyes’ |
| 111 | Alnus spaethii | 813 | Pinus nigra ‘Obelisk’ |
| 112 | Alstroemeria | 814 | Pinus nigra var. austriaca |
| 113 | Amelanchier | 815 | Pinus peuce |
| 114 | Amelanchier alnifolia ‘Obelisk’ | 816 | Pinus pinaster |
| 115 | Amelanchier canadensis | 817 | Pinus pungens glauca |
| 116 | Amelanchier canadensis ‘Glenform Rainbow Pillar’ | 818 | Pinus radiate |
| 117 | Amelanchier ‘Edelweiss’ | 819 | Pinus radiata ‘Aurea’ |
| 118 | Amelanchier grandiflora ‘Ballerina’ | 820 | Pinus strobus ‘Minima’ |
| 119 | Amelanchier ‘La Paloma’ | 821 | Pinus strobus ‘Tiny Kurls’ |
| 120 | Amelanchier laevis ‘R J Hilton’ | 822 | Pinus sylvestris |
| 121 | Amelanchier laevis ‘Snowflakes’ | 823 | Pinus sylvestris ‘Chantry Blue’ |
| 122 | Amelanchier lamarckii | 824 | Pinus sylvestris ‘Gold Medal’ |
| 123 | Amelanchier lamarckii ‘Robin Hill’ | 825 | Pinus sylvestris ‘Westonbirt’ |
| 124 | Amelanchier ‘Northline’ | 826 | Pinus thunbergii ‘Banshosho’ |
| 125 | Amelanchier × grandiflora ‘Ballerina’ | 827 | Pinus wallichiana |
| 126 | Amelanchier × grandiflora ‘Robin Hill’ | 828 | Pinus × holdfordiana |
| 127 | Ammonophylla | 829 | Pittosporum |
| 128 | Anemanthele | 830 | Platanus |
| 129 | Anemone | 831 | Platanus orientalis digitalis |
| 130 | Aquilegia | 832 | Platanus × hispanica |
| 131 | Araucaria araucana | 833 | Platanus × hispanica ‘Louisa Lead’ |
| 132 | Arbutus | 834 | Polemonium |
| 133 | Arbutus unedo | 835 | Polygonatum |
| 134 | Armeria | 836 | Polypodium |
| 135 | Artemisia | 837 | Polystichum |
| 136 | Arum | 838 | Populus |
| 137 | Aruncus | 839 | Populus nigra |
| 138 | Asplenium | 840 | Populus nigra ‘Italica’ |
| 139 | Astelia | 841 | Populus tremula |
| 140 | Aster | 842 | Potentilla |
| 141 | Astilbe | 843 | Primula |
| 142 | Astrantia | 844 | Prunus |
| 143 | Athyrium | 845 | Prunus × subhirtella ‘Autumnalis’ |
| 144 | Aucuba | 846 | Prunus × subhirtella ‘Autumnalis Rosea’ |
| 145 | Baptisia | 847 | Prunus × subhirtella ‘Pendula Plena Rosea’ |
| 146 | Berberis | 848 | Prunus ‘Accolade’ |
| 147 | Berberis darwinii | 849 | Prunus ‘Amanogawa’ |
| 148 | Berberis thunbergii | 850 | Prunus ‘Amber Heart’ |
| 149 | Berberis thunbergii f. atropurpurea | 851 | Prunus ‘Aprikyra’ |
| 150 | Bergenia | 852 | Prunus ‘Aprimira’ |
| 151 | Betula | 853 | Prunus ‘Aprisali’ |
| 152 | Betula alba pendula | 854 | Prunus ‘Areko’ |
| 153 | Betula albosinensis ‘Chinese Ruby’ | 855 | Prunus armeniaca ‘Aviera’ |
| 154 | Betula albosinensis ‘Fascination’ | 856 | Prunus armeniaca ‘Bergeron’ |
| 155 | Betula albosinensis ‘Hillier’ | 857 | Prunus armeniaca ‘Bergeval’ |
| 156 | Betula albosinensis ‘Red Panda’ | 858 | Prunus armeniaca ‘Compacta’ |
| 157 | Betula costata ‘Daleside’ | 859 | Prunus armeniaca ‘Garden Aprigold’ |
| 158 | Betula ‘Edinburgh’ | 860 | Prunus armeniaca ‘Goldcot’ |
| 159 | Betula ermanii | 861 | Prunus armeniaca ‘Golden Glow’ |
| 160 | Betula ermanii ‘Mount Zao Purple’ | 862 | Prunus armeniaca ‘Kioto’ |
| 161 | Betula ermanii ‘Polar Bear’ | 863 | Prunus armeniaca ‘Pink Marry’ |
| 162 | Betula ermanii ‘White Chocolate’ | 864 | Prunus armeniaca ‘Robada’ |
| 163 | Betula ‘Fascination’ | 865 | Prunus armeniaca ‘Tomcot’ |
| 164 | Betula ‘Fetisowii’ | 866 | Prunus ‘Asano’ |
| 165 | Betula lenta | 867 | Prunus ‘Athos’ |
| 166 | Betula nigra | 868 | Prunus avium |
| 167 | Betula nigra ‘Shiloh Splash’ | 869 | Prunus avium ‘Plena’ |
| 168 | Betula papyrifera var. kenaica | 870 | Prunus ‘Beni‐yutaka’ |
| 169 | Betula pendula | 871 | Prunus ‘Black Oliver’ |
| 170 | Betula pendula ‘Dalecarlica’ | 872 | Prunus ‘Blushing Bride’ |
| 171 | Betula pendula ‘Fastigiata Joes’ | 873 | Prunus ‘Burcombe’ |
| 172 | Betula pendula fastigiata ‘Obelisk’ | 874 | Prunus campanulata |
| 173 | Betula pendula ‘Royal Frost’ | 875 | Prunus ‘Candy Floss’ |
| 174 | Betula pendula ‘Spider Alley’ | 876 | Prunus ‘Catherine’ |
| 175 | Betula pendula ‘Tristis’ | 877 | Prunus ‘Celeste’ |
| 176 | Betula pendula ‘Youngii’ | 878 | Prunus cerasifera |
| 177 | Betula pendula ‘Zwitsers Glory’ | 879 | Prunus cerasifera ‘Crimson Pointe’ |
| 178 | Betula pubsecens | 880 | Prunus cerasifera ‘Nigra’ |
| 179 | Betula utilis ‘Cinnamon’ | 881 | Prunus cerasifera ‘Pissardii’ |
| 180 | Betula utilis ‘Dark‐Ness’ | 882 | Prunus ‘Chocolate Ice’ |
| 181 | Betula utilis ‘Edinburgh’ | 883 | Prunus ‘Collingwood Ingram’ |
| 182 | Betula utilis ‘Jermyns’ | 884 | Prunus ‘Countess’ |
| 183 | Betula utilis ‘Melony Sanders’ | 885 | Prunus ‘Daikoku’ |
| 184 | Betula utilis ‘Moonbeam’ | 886 | Prunus ‘de Nancy’ |
| 185 | Betula utilis ‘Mount Luoji’ | 887 | Prunus domestica ‘Avalon’ |
| 186 | Betula utilis ‘Snow Queen’ | 888 | Prunus domestica ‘Belle de Louvain’ |
| 187 | Betula utilis subsp. albosinensis ‘Cacao’ | 889 | Prunus domestica ‘Blaisdon Red’ |
| 188 | Betula utilis subsp. albosinensis ‘China Rose’ | 890 | Prunus domestica ‘Blue Tit’ |
| 189 | Betula utilis subsp. albosinensis ‘Hergest’ | 891 | Prunus domestica ‘Cambridge’ |
| 190 | Betula utilis subsp. albosinensis ‘Kansu’ | 892 | Prunus domestica ‘Coes Golden Drop’ |
| 191 | Betula utilis subsp. albosinensis ‘Pink Champagne’ | 893 | Prunus domestica ‘Czar’ |
| 192 | Betula utilis subsp. albosinensis ‘Red Panda’ | 894 | Prunus domestica ‘Denniston's Superb’ |
| 193 | Betula utilis var. jacquemontii | 895 | Prunus domestica ‘Early Transparent’ |
| 194 | Betula utilis var. jacquemontii ‘Grayswood Ghost’ | 896 | Prunus domestica ‘Edda’ |
| 195 | Betula utilis var. jacquemontii ‘Jermyns’ | 897 | Prunus domestica ‘Excalibur’ |
| 196 | Betula utilis var. jacquemontii ‘McBeath’ | 898 | Prunus domestica ‘Ferbleue’ |
| 197 | Betula utilis var. jacquemontii ‘Silver Shadow’ | 899 | Prunus domestica ‘Gordon Castle’ |
| 198 | Betula utilis var. jacquemontii ‘Trinity College’ | 900 | Prunus domestica ‘Guinevere’ |
| 199 | Betula utilis ‘Wakehurst Place Chocolate’ | 901 | Prunus domestica ‘Haganta’ |
| 200 | Blechnum | 902 | Prunus domestica ‘Herman’ |
| 201 | Brachyglottis | 903 | Prunus domestica ‘Jefferson’ |
| 202 | Brunnera | 904 | Prunus domestica ‘Jubilee’ |
| 203 | Buddleja | 905 | Prunus domestica ‘Katinka’ |
| 204 | Buxus | 906 | Prunus domestica ‘Lindsey Gage’ |
| 205 | Buxus sempervirens | 907 | Prunus domestica ‘Malling Elizabeth’ |
| 206 | Calamagrostis | 908 | Prunus domestica ‘Marjorie's Seedling’ |
| 207 | Callicarpa bodinieri ‘Profusion’ | 909 | Prunus domestica ‘Meritare’ |
| 208 | Calluna | 910 | Prunus domestica ‘Old Green Gage’ |
| 209 | Calycanthus ‘Aphrodite’ | 911 | Prunus domestica ‘Opal’ |
| 210 | Campanula | 912 | Prunus domestica ‘Oullins Golden’ |
| 211 | Carex | 913 | Prunus domestica ‘Purple Pershore’ |
| 212 | Carpinus | 914 | Prunus domestica ‘Queen's Crown’ |
| 213 | Carpinus betulus | 915 | Prunus domestica ‘Reeves’ |
| 214 | Carpinus betulus ‘Chartreuse’ | 916 | Prunus domestica ‘Reine Claude de Bavay’ |
| 215 | Carpinus betulus ‘Fastigiata’ | 917 | Prunus domestica ‘River's Early Prolific’ |
| 216 | Carpinus betulus ‘Frans Fontaine’ | 918 | Prunus domestica ‘Sanctus Hubertus’ |
| 217 | Carpinus betulus ‘Lucas’ | 919 | Prunus domestica ‘Seneca’ |
| 218 | Carpinus betulus ‘Rockhampton Red’ | 920 | Prunus domestica ‘Stella's Star’ |
| 219 | Carpinus betulus ‘Streetwise’ | 921 | Prunus domestica subsp. insititia ‘Aylesbury Prune’ |
| 220 | Caryopteris | 922 | Prunus domestica subsp. insititia ‘Farleigh’ |
| 221 | Castanea | 923 | Prunus domestica subsp. insititia ‘King of the Damsons’ |
| 222 | Castanea sativa | 924 | Prunus domestica subsp. insititia ‘Merryweather’ |
| 223 | Castanea sativa ‘Anny's Summer Red’ | 925 | Prunus domestica subsp. insititia ‘Shepherds Bullace’ |
| 224 | Catalpa bignoniodes | 926 | Prunus domestica subsp. insititia ‘Shropshire Prune’ |
| 225 | Catalpa bignoniodes ‘Aurea’ | 927 | Prunus domestica subsp. insititia ‘Sweet Prune’ |
| 226 | Catalpa × erubescens ‘Purpurea’ | 928 | Prunus domestica ‘Swan’ |
| 227 | Ceanothus | 929 | Prunus domestica ‘Topend Plus’ |
| 228 | Ceanothus arboreus ‘Trewithen Blue’ | 930 | Prunus domestica ‘Topfive’ |
| 229 | Cedrus atlantica | 931 | Prunus domestica ‘Tophit Plus’ |
| 230 | Cedrus atlantica ‘Glauca’ | 932 | Prunus domestica ‘Toptaste Kulinaria’ |
| 231 | Cedrus atlantica ‘Glauca Pendula’ | 933 | Prunus domestica ‘Victoria’ |
| 232 | Cedrus deodara | 934 | Prunus domestica ‘Violet’ |
| 233 | Cedrus deodara ‘Karl Fuchs’ | 935 | Prunus domestica ‘Warwickshire Drooper’ |
| 234 | Cedrus deodara ‘Klondyke’ | 936 | Prunus domestica ‘Willingham’ |
| 235 | Cedrus libani | 937 | Prunus domestica ‘Yellow Pershore’ |
| 236 | Celtis australis | 938 | Prunus ‘Early Red Maraly’ |
| 237 | Centaurea | 939 | Prunus ‘Fertile’ |
| 238 | Centranthus | 940 | Prunus ‘Fice’ |
| 239 | Ceratostigma | 941 | Prunus ‘'Flavor King |
| 240 | Cercidiphyllum japonicum | 942 | Prunus ‘Folfer’ |
| 241 | Cercidiphyllum japonicum ‘Pendulum’ | 943 | Prunus ‘Fragrant Cloud’ |
| 242 | Cercis canadensis | 944 | Prunus ‘Frilly Frock’ |
| 243 | Cercis canadensis ‘Alley Cat’ | 945 | Prunus ‘Fugenzo’ |
| 244 | Cercis canadensis ‘Carolina Sweetheart’ | 946 | Prunus ‘Golden Sphere’ |
| 245 | Cercis canadensis ‘Eternal Flame’ | 947 | Prunus ‘Gyoiko’ |
| 246 | Cercis canadensis ‘Forest Pansy’ | 948 | Prunus ‘Gypsy’ |
| 247 | Cercis canadensis ‘Golden Falls’ | 949 | Prunus ‘Hally Jolivette’ |
| 248 | Cercis canadensis ‘Hearts of Gold’ | 950 | Prunus ‘Henriette’ |
| 249 | Cercis canadensis ‘Lavender Twist’ | 951 | Prunus ‘Hertford’ |
| 250 | Cercis canadensis ‘Merlot’ | 952 | Prunus ‘Hokusai’ |
| 251 | Cercis canadensis ‘Pink Pom Pom’ | 953 | Prunus ‘Horinji’ |
| 252 | Cercis canadensis ‘Rising Sun’ | 954 | Prunus ‘Ichiyo’ |
| 253 | Cercis canadensis ‘Ruby Falls’ | 955 | Prunus incisa ‘Kojo‐no‐mai’ |
| 254 | Cercis canadensis ‘Vanilla Twist’ | 956 | Prunus incisa ‘Mikinori’ |
| 255 | Cercis chinensis ‘Avondale’ | 957 | Prunus incisa ‘Oshidori PRINCESSE’ |
| 256 | Cercis chinensis ‘Diane’ | 958 | Prunus incisa ‘Pendula’ |
| 257 | Cercis reniformis ‘Oklahoma’ | 959 | Prunus incisa ‘Praecox’ |
| 258 | Cercis reniformis ‘Texan White’ | 960 | Prunus incisa ‘Yamadei’ |
| 259 | Cercis silaquastrum | 961 | Prunus ‘Ingrid’ |
| 260 | Cercis silaquastrum ‘Bodnant’ | 962 | Prunus ‘Jacqueline’ |
| 261 | Chaenomeles | 963 | Prunus ‘Kanzan’ |
| 262 | Chamaecyparis | 964 | Prunus Ki 2004 R11 B93 |
| 263 | Chamaecyparis lawsoniana | 965 | Prunus Ki 2004 R14 B56 |
| 264 | Choisya | 966 | Prunus ‘Kiku‐shidare‐zakura’ |
| 265 | Cistus | 967 | Prunus ‘KIR LAMOUR’ |
| 266 | Cladrastis kentuckea | 968 | Prunus ‘KIR ROSSO’ |
| 267 | Clematis | 969 | Prunus ‘KIR VULCANO’ |
| 268 | Convolvulus | 970 | Prunus ‘Knights Early Black’ |
| 269 | Coprosma | 971 | Prunus ‘Kofugen’ |
| 270 | Coreopsis | 972 | Prunus ‘Kordia’ |
| 271 | Cornus | 973 | Prunus ‘Kursar’ |
| 272 | Cornus kousa var. chinensis | 974 | Prunus ‘Lapins Cherokee’ |
| 273 | Cornus sanguinea | 975 | Prunus laurocerasus |
| 274 | Cortaderia | 976 | Prunus laurocerasus ‘Magnoliifolia’ |
| 275 | Corydalis | 977 | Prunus laurocerasus ‘Rotund’ |
| 276 | Corylus | 978 | Prunus litigiosa |
| 277 | Corylus avellana | 979 | Prunus ‘Litigiosa’ |
| 278 | Corylus avellana ‘Contorta’ | 980 | Prunus ‘Little Pink Perfection’ |
| 279 | Corylus avellana ‘Gunslebert’ | 981 | Prunus lusitanica |
| 280 | Corylus avellana ‘Hall's Giant’ | 982 | Prunus maackii ‘Amber Beauty’ |
| 281 | Corylus avellana ‘Lang Tidlig Zeller’ | 983 | Prunus ‘Merchant’ |
| 282 | Corylus avellana ‘Nottingham’ | 984 | Prunus ‘Merton Glory’ |
| 283 | Corylus avellana ‘Tonda Di Giffoni’ | 985 | Prunus ‘Mikurama‐gaeshi’ |
| 284 | Corylus avellana ‘Tonda Gentile de le Romana’ | 986 | Prunus ‘Morello’ |
| 285 | Corylus avellana ‘Tonda Gentile Trilobata’ | 987 | Prunus ‘Mount Fuji’ |
| 286 | Corylus avellana ‘Webbs Prize Cob’ | 988 | Prunus ‘Nabella’ |
| 287 | Corylus colurna | 989 | Prunus ‘Napoleon Bigarreau’ |
| 288 | Corylus ‘Cosford’ | 990 | Prunus ‘Nimba’ |
| 289 | Corylus ‘Red Filbert’ | 991 | Prunus ‘Okame’ |
| 290 | Corylus ‘Te‐Terra Red’ | 992 | Prunus padus |
| 291 | Cosmos | 993 | Prunus padus ‘Le Thoureil’ |
| 292 | Cotinus | 994 | Prunus padus ‘Select’ |
| 293 | Cotoneaster | 995 | Prunus ‘Pandora’ |
| 294 | Cotoneaster × suecicus ‘Coral Beauty’ | 996 | Prunus ‘Papillon’ |
| 295 | Cotoneaster bullatus | 997 | Prunus pendula ‘Ascendens Rosea’ |
| 296 | Cotoneaster franchettii | 998 | Prunus pendula ‘Pendula Rubra’ |
| 297 | Cotoneaster frigidus ‘Cornubia’ | 999 | Prunus pendula ‘Stellata’ |
| 298 | Cotoneaster horizontalis | 1000 | Prunus ‘Penny’ |
| 299 | Cotoneaster ‘Hybridus Pendulus’ | 1001 | Prunus persica ‘Amsden June’ |
| 300 | Cotoneaster lacteus | 1002 | Prunus persica ‘Avalon Pride’ |
| 301 | Cotoneaster salicifolius ‘Exburiensis’ | 1003 | Prunus persica ‘Garden Beauty’ |
| 302 | Cotoneaster salicifolius ‘Repens’ | 1004 | Prunus persica ‘Garden Lady’ |
| 303 | Cotoneaster simonsii | 1005 | Prunus persica ‘Gorgeous’ |
| 304 | Cotoneaster × suecicus ‘Juliette’ | 1006 | Prunus persica ‘Hales Early’ |
| 305 | Crataegus | 1007 | Prunus persica ‘Lord Napier’ |
| 306 | Crataegus azarolus | 1008 | Prunus persica ‘Mesembrine’ |
| 307 | Crataegus laevigata ‘Crimson Cloud’ | 1009 | Prunus persica ‘Nectarella’ |
| 308 | Crataegus laevigata ‘Pauls Scarlet’ | 1010 | Prunus persica ‘Peregrine’ |
| 309 | Crataegus laevigata ‘Plena’ | 1011 | Prunus persica ‘Pineapple’ |
| 310 | Crataegus laevigata ‘Rosea Flore Pleno’ | 1012 | Prunus persica ‘Red Haven’ |
| 311 | Crataegus lavallei ‘Carreri’ | 1013 | Prunus persica ‘Rochester’ |
| 312 | Crataegus monogyna | 1014 | Prunus persica ‘Saturn’ |
| 313 | Crataegus monogyna ‘Stricta’ | 1015 | Prunus persica ‘Terrace Amber’ |
| 314 | Crataegus persimilis ‘Prunifolia’ | 1016 | Prunus ‘Petit Noir’ |
| 315 | Crataegus persimilis ‘Prunifolia Splendens’ | 1017 | Prunus ‘Pink Parasol’ |
| 316 | Crataegus pinnatifida var. major ‘Big Golden Star’ | 1018 | Prunus ‘Pink Perfection’ |
| 317 | Crataegus schraderiana | 1019 | Prunus ‘Pink Shell’ |
| 318 | Crataegus succulenta ‘Jubilee’ | 1020 | Prunus ‘Powder Puff’ |
| 319 | Crataegus × dippeliana | 1021 | Prunus ‘Regina’ |
| 320 | Crataegus × lavalleei ‘Carrierei’ | 1022 | Prunus ‘Robijn’ |
| 321 | Crocosmia | 1023 | Prunus ‘Roundel Heart’ |
| 322 | Cryptomeria japonica | 1024 | Prunus ‘Royal Burgundy’ |
| 323 | Cryptomeria japonica ‘Gracilis’ | 1025 | Prunus ‘Royal Flame’ |
| 324 | Cryptomeria japonica ‘Sekkan‐sugi’ | 1026 | Prunus ‘Ruby COLUMNAR’ |
| 325 | Cupressocyparis | 1027 | Prunus rufa |
| 326 | Cupressocyparis leylandii | 1028 | Prunus sargentii |
| 327 | Cupressus | 1029 | Prunus sargentii ‘Rancho’ |
| 328 | Cupressus glabra ‘Blue Ice’ | 1030 | Prunus serrula |
| 329 | Cupressus macrocarpa | 1031 | Prunus serrula ‘Branklyn’ |
| 330 | Cupressus macrocarpa ‘Wilma’ | 1032 | Prunus ‘Shirofugen’ |
| 331 | Cupressus sempervirens ‘Totem’ | 1033 | Prunus ‘Shirotae’ |
| 332 | Cydonia ‘Aromatnaya’ | 1034 | Prunus ‘Shosar’ |
| 333 | Cydonia ‘Bereczki’ | 1035 | Prunus ‘Skeena’ |
| 334 | Cydonia ‘Isfahan’ | 1036 | Prunus ‘Snow Goose’ |
| 335 | Cydonia ‘Meech's Prolific’ | 1037 | Prunus ‘Snow Showers’ |
| 336 | Cydonia ‘Serbian Gold’ | 1038 | Prunus spinosa |
| 337 | Cydonia ‘Vranja’ | 1039 | Prunus ‘Spire’ |
| 338 | Cynoglossum | 1040 | Prunus ‘Spring Snow’ |
| 339 | Cytisus | 1041 | Prunus ‘STARDUST COVEU’ |
| 340 | Dahlia | 1042 | Prunus ‘Stella’ |
| 341 | Daphne | 1043 | Prunus ‘Summer Sun’ |
| 342 | Davidia involucrata | 1044 | Prunus ‘Sunburst’ |
| 343 | Davidia involucrata ‘Sonoma’ | 1045 | Prunus ‘Sunset Boulevard’ |
| 344 | Delosperma | 1046 | Prunus ‘Sweetheart’ |
| 345 | Delphinium | 1047 | Prunus ‘Sylvia’ |
| 346 | Deschampsia | 1048 | Prunus ‘Tai‐haku’ |
| 347 | Deutzia | 1049 | Prunus ‘Taoyame’ |
| 348 | Dicentra | 1050 | Prunus ‘The Bride’ |
| 349 | Diervilla | 1051 | Prunus ‘Tiltstone Hellfire’ |
| 350 | Digitalis | 1052 | Prunus ‘Trailblazer’ |
| 351 | Doronicum | 1053 | Prunus ‘Ukon’ |
| 352 | Dryopteris | 1054 | Prunus ‘Vanda’ |
| 353 | Echinacea | 1055 | Prunus ‘Walter’ |
| 354 | Echinops | 1056 | Prunus ‘Waterloo’ |
| 355 | Elaeagnus | 1057 | Prunus ‘Weeping Yoshino’ |
| 356 | Elaeagnus ‘Quicksilver’ | 1058 | Prunus × persicoides ‘Spring Glow’ |
| 357 | Epimedium | 1059 | Prunus × schmittii |
| 358 | Eremurus | 1060 | Prunus × yedoensis |
| 359 | Erigeron | 1061 | Pseudotsuga menziesii |
| 360 | Eriophorum | 1062 | Pterocarya stenoptera ‘Fern Leaf’ |
| 361 | Eriostemon | 1063 | Pulmonaria |
| 362 | Eryngium | 1064 | Pyracantha |
| 363 | Erysimum | 1065 | Pyrus |
| 364 | Escallonia | 1066 | Pyrus ‘Barnet’ |
| 365 | Eucalyptus | 1067 | Pyrus ‘Benita Rafzas’ |
| 366 | Eucalyptus ‘Azura’ | 1068 | Pyrus ‘Beth’ |
| 367 | Eucalyptus glaucescens | 1069 | Pyrus ‘Beurre Hardy’ |
| 368 | Eucalyptus gunnii | 1070 | Pyrus ‘Beurre Superfin’ |
| 369 | Euonymus | 1071 | Pyrus ‘Black Worcester’ |
| 370 | Euonymus alatus ‘Compactus’ | 1072 | Pyrus ‘Blakeney Red’ |
| 371 | Euonymus clivicola | 1073 | Pyrus ‘Brandy’ |
| 372 | Euonymus europaeus | 1074 | Pyrus calleryana ‘Chanticleer’ |
| 373 | Euonymus europaeus ‘Brilliant’ | 1075 | Pyrus calleryana ‘Red Spire’ |
| 374 | Euonymus europaeus ‘Red Cascade’ | 1076 | Pyrus ‘Catillac’ |
| 375 | Euonymus hamiltonianus ‘Indian Summer’ | 1077 | Pyrus ‘Celebration NUVAR’ |
| 376 | Euonymus hamiltonianus ‘Koi Boy’ | 1078 | Pyrus ‘Christie’ |
| 377 | Euonymus japonicus ‘Bravo’ | 1079 | Pyrus ‘Comice’ |
| 378 | Euonymus phellomanus | 1080 | Pyrus communis |
| 379 | Euonymus planipes | 1081 | Pyrus ‘Concorde’ |
| 380 | Euonymus planipes ‘Sancho’ | 1082 | Pyrus ‘Conference’ |
| 381 | Euphorbia | 1083 | Pyrus ‘Conference Moors Giant’ |
| 382 | Exochorda | 1084 | Pyrus ‘Doyenne du Comice’ |
| 383 | Exochorda × macrantha ‘The Bride’ | 1085 | Pyrus elaeagrifolia ‘Silver Sails’ |
| 384 | Fagus | 1086 | Pyrus ‘Fondante d'Automne’ |
| 385 | Fagus aspelenifolia | 1087 | Pyrus ‘Gin’ |
| 386 | Fagus sylvatica | 1088 | Pyrus ‘Glou Morceau’ |
| 387 | Fagus sylvatica ‘Atropurpurea’ | 1089 | Pyrus ‘Gorham’ |
| 388 | Fagus sylvatica ‘Black Swan’ | 1090 | Pyrus ‘Green Horse’ |
| 389 | Fagus sylvatica ‘Dawyck’ | 1091 | Pyrus ‘Hellens Early’ |
| 390 | Fagus sylvatica ‘Dawyck Gold’ | 1092 | Pyrus ‘Hendre Huffcap’ |
| 391 | Fagus sylvatica ‘Dawyck Purple’ | 1093 | Pyrus ‘Humbug’ |
| 392 | Fagus sylvatica ‘Midnight Feather’ | 1094 | Pyrus ‘Invincible delwinor fertilia’ |
| 393 | Fagus sylvatica ‘Pendula’ | 1095 | Pyrus ‘Jargonelle’ |
| 394 | Fagus sylvatica ‘Purple Fountain’ | 1096 | Pyrus ‘Josephine de Malines’ |
| 395 | Fagus sylvatica ‘Purpurea’ | 1097 | Pyrus ‘Judge Amphlet’ |
| 396 | Fagus sylvatica ‘Purpurea Pendula’ | 1098 | Pyrus ‘Kumoi’ |
| 397 | Fagus sylvatica ‘Purpurea Tricolor | 1099 | Pyrus ‘Louise Bonne of Jersey’ |
| 398 | Fagus sylvatica ‘Riversii’ | 1100 | Pyrus ‘Merton Pride’ |
| 399 | Fagus sylvatica var. heterophylla ‘Asplenifolia’ | 1101 | Pyrus ‘Moonglow’ |
| 400 | Fargesia | 1102 | Pyrus ‘Obelisk’ |
| 401 | Fatsia | 1103 | Pyrus ‘Olympic’ |
| 402 | Festuca | 1104 | Pyrus ‘Onward’ |
| 403 | Ficus ‘Brown Turkey’ | 1105 | Pyrus ‘Packham's Triumph’ |
| 404 | Ficus ‘Dalmatie’ | 1106 | Pyrus ‘Pitmaston Dutchess’ |
| 405 | Ficus ‘Ice Crystal’ | 1107 | Pyrus ‘Red Pear’ |
| 406 | Ficus ‘Little Miss Figgy’ | 1108 | Pyrus salicifolia ‘Pendula’ |
| 407 | Ficus ‘Panache’ | 1109 | Pyrus ‘Sensation’ |
| 408 | Filipendula | 1110 | Pyrus ‘Shinseiki’ |
| 409 | Foeniculum | 1111 | Pyrus ‘Shipover’ |
| 410 | Forsythia | 1112 | Pyrus ‘Thorn’ |
| 411 | Forsythia suspensa ‘Nymans’ | 1113 | Pyrus ‘Williams’ |
| 412 | Forsythia × intermedia ‘Lynwood’ | 1114 | Pyrus ‘Williams’ Bon Chrétien’ |
| 413 | Fraxinus americana | 1115 | Pyrus ‘Winnal's Longdon’ |
| 414 | Fraxinus angustifolia | 1116 | Pyrus ‘Winter Nelis’ |
| 415 | Fraxinus ornus ‘Obelisk’ | 1117 | Pyrus ‘Yellow Huffcap’ |
| 416 | Fuchsia | 1118 | Quercus |
| 417 | Galium | 1119 | Quercus castaneifolia ‘Green Spire’ |
| 418 | Garrya | 1120 | Quercus cerris |
| 419 | Gaultheria procumbens | 1121 | Quercus frainetto ‘Hungarian Crown’ |
| 420 | Gaultheria shallon | 1122 | Quercus ilex |
| 421 | Gaura | 1123 | Quercus myrsinifolia |
| 422 | Genista | 1124 | Quercus palustris |
| 423 | Geranium | 1125 | Quercus palustris ‘Green Pillar’ |
| 424 | Geum | 1126 | Quercus petraea |
| 425 | Ginkgo biloba | 1127 | Quercus robur |
| 426 | Ginkgo biloba ‘Blagon’ | 1128 | Quercus robur ‘Fastigiata Koster’ |
| 427 | Ginkgo biloba ‘Globosum’ | 1129 | Quercus rubra |
| 428 | Ginkgo biloba ‘Menhir’ | 1130 | Quercus texana ‘New Madrid’ |
| 429 | Ginkgo biloba ‘Saratoga’ | 1131 | Quercus × bimundorum ‘Crimson Spire’ |
| 430 | Gleditsia triacanthos ‘Skyline’ | 1132 | Quercus × warei ‘Regal Prince’ |
| 431 | Gleditsia triacanthos ‘Sunburst’ | 1133 | Rhamnus |
| 432 | Griselinia | 1134 | Rhamnus cathartica |
| 433 | Hakonechloa | 1135 | Rhamnus frangula |
| 434 | Halesia carolina | 1136 | Rheum ‘Strawberry Surprise’ |
| 435 | Halimium | 1137 | Rheum ‘Timperley Early’ |
| 436 | Hamamelis × intermedia ‘Arnold Promise’ | 1138 | Rheum ‘Victoria’ |
| 437 | Hamamelis × intermedia ‘Diane’ | 1139 | Rhus |
| 438 | Hamamelis × intermedia ‘Jelena’ | 1140 | Ribes |
| 439 | Hamamelis × intermedia ‘Pallida’ | 1141 | Ribes ‘Ben Connan’ |
| 440 | Hebe | 1142 | Ribes ‘Ben Sarek’ |
| 441 | Hedera | 1143 | Ribes ‘Black ‘n’ Red Premiere’ |
| 442 | Helenium | 1144 | Ribes ‘Blackbells’ |
| 443 | Helichrysum | 1145 | Ribes ‘Blanka’ |
| 444 | Helleborus | 1146 | Ribes ‘Captivator’ |
| 445 | Hemerocallis | 1147 | Ribes ‘Hinnonmaki Red’ |
| 446 | Heptacodium miconioides | 1148 | Ribes ‘Hinnonmaki Yellow’ |
| 447 | Heuchera | 1149 | Ribes ‘Invicta’ |
| 448 | Heucherella | 1150 | Ribes ‘Jonkheer van Tets’ |
| 449 | Hippophae | 1151 | Ribes ‘Junifer’ |
| 450 | Hippophae rhamnoides | 1152 | Ribes ‘Lowberry Little Black Sugar’ |
| 451 | Hippophae salicifolia ‘Streetwise’ | 1153 | Ribes ‘Mucurines’ |
| 452 | Hoheria sexstylosa ‘Snow White’ | 1154 | Ribes ‘Ojebyn’ |
| 453 | Hosta | 1155 | Ribes ‘Rovada’ |
| 454 | Houttuynia | 1156 | Ribes ‘Titania’ |
| 455 | Hydrangea | 1157 | Robinia |
| 456 | Hypericum | 1158 | Robinia ‘Bessoniana’ |
| 457 | Iberis | 1159 | Robinia ‘Casque Rouge’ |
| 458 | Ilex | 1160 | Robinia pseudoacacia |
| 459 | Ilex aquifolium | 1161 | Robinia pseudoacacia ‘Frisia’ |
| 460 | Ilex aquifolium ‘Alaska’ | 1162 | Robinia pseudoacacia ‘Lace Lady Twisty Babe’ |
| 461 | Ilex aquifolium ‘Argentea Marginata’ | 1163 | Robinia × margaretta ‘Pink Cascade’ |
| 462 | Ilex aquifolium ‘Handsworth New Silver’ | 1164 | Rosa |
| 463 | Ilex aquifolium ‘J.C. van Tol’ | 1165 | Rosa arvensis |
| 464 | Ilex aquifolium ‘Marijo’ | 1166 | Rosa canina |
| 465 | Ilex aquifolium ‘Nellie R Stevens’ | 1167 | Rosa rubiginosa |
| 466 | Ilex crenata | 1168 | Rosa rugosa |
| 467 | Ilex × altaclarensis ‘James G. Esson’ | 1169 | Rosa rugosa ‘Alba’ |
| 468 | Ilex × altaclerensis ‘Golden King’ | 1170 | Rosa rugosa rubra |
| 469 | Ilex × Koehneana ‘Chestnut Leaf’ | 1171 | Rosa spinosissima |
| 470 | Imperata | 1172 | Rosmarinus |
| 471 | Iris | 1173 | Rubus ‘Allgold’ |
| 472 | Jasminum | 1174 | Rubus ‘Autumn Bliss’ |
| 473 | Juglans ‘Apollo’ | 1175 | Rubus ‘Buckingham’ |
| 474 | Juglans ‘Broadview’ | 1176 | Rubus ‘Cascade Delight’ |
| 475 | Juglans ‘Buccaneer’ | 1177 | Rubus fruticosus ‘Arapaho’ |
| 476 | Juglans ‘Chandler’ | 1178 | Rubus fruticosus ‘Loch Ness’ |
| 477 | Juglans ‘Fernette’ | 1179 | Rubus fruticosus ‘Lowberry Little Black Prince’ |
| 478 | Juglans ‘Fernor’ | 1180 | Rubus fruticosus ‘Navaho Summerlong’ |
| 479 | Juglans ‘Franquette’ | 1181 | Rubus fruticosus ‘Oregon Thornless’ |
| 480 | Juglans ‘Mars’ | 1182 | Rubus ‘Glen Ample’ |
| 481 | Juglans nigra | 1183 | Rubus ‘Glen Carron’ |
| 482 | Juglans regia | 1184 | Rubus ‘Golden Everest’ |
| 483 | Juniperus | 1185 | Rubus ‘Joan J’ |
| 484 | Juniperus communis | 1186 | Rubus ‘Lowberry Goodasgold’ |
| 485 | Juniperus scopulorum ‘Blue Arrow’ | 1187 | Rubus ‘Lowberry Little Sweet Sister’ |
| 486 | Knautia | 1188 | Rubus ‘Malling Juno’ |
| 487 | Kniphofia | 1189 | Rubus ‘Octavia’ |
| 488 | Koelreuteria paniculata | 1190 | Rubus ‘Thornfree’ |
| 489 | Koelreuteria paniculata ‘Coral Sun’ | 1191 | Rubus ‘Tulameen’ |
| 490 | Laburnum | 1192 | Rudbeckia |
| 491 | Laburnum anagyroides | 1193 | Salix |
| 492 | Laburnum anagyroides ‘Yellow Rocket’ | 1194 | Salix alba |
| 493 | Lamium | 1195 | Salix alba ‘Britzensis’ |
| 494 | Larix | 1196 | Salix aurita |
| 495 | Larix decidua | 1197 | Salix babylonica pendula |
| 496 | Larix kaempferi | 1198 | Salix caprea |
| 497 | Larix × decidua | 1199 | Salix caprea ‘Pendula’ |
| 498 | Larix × eurolepsis | 1200 | Salix cinerea |
| 499 | Lavandula | 1201 | Salix erythroflexuosa ‘Golden Curls’ |
| 500 | Lavatera | 1202 | Salix ‘Hakuro Nishiki’ |
| 501 | Leucanthemum | 1203 | Salix pentandra |
| 502 | Leucothoe | 1204 | Salix viminalis |
| 503 | Leycesteria | 1205 | Salvia |
| 504 | Leymus | 1206 | Sambucus |
| 505 | Liatris | 1207 | Sambucus nigra |
| 506 | Ligularia | 1208 | Sambucus nigra ‘Black Tower Eiffel’ |
| 507 | Ligustrum | 1209 | Sambucus nigra porphyrophylla ‘Black Beauty’ |
| 508 | Ligustrum ovalifolium | 1210 | Sambucus nigra porphyrophylla ‘Black Lace’ |
| 509 | Ligustrum ovalifolium ‘Aureum’ | 1211 | Sambucus ‘Sampo’ |
| 510 | Ligustrum vulgare | 1212 | Sanguisorba |
| 511 | Liquidambar | 1213 | Santolina |
| 512 | Liquidambar styraciflua | 1214 | Sarcococca confusa |
| 513 | Liquidambar styraciflua ‘Lane Roberts’ | 1215 | Scabiosa |
| 514 | Liquidambar styraciflua ‘Palo Alto’ | 1216 | Schizostylis |
| 515 | Liquidambar styraciflua ‘Slender Silhouette’ | 1217 | Sedum |
| 516 | Liquidambar styraciflua ‘Stared’ | 1218 | Senecio |
| 517 | Liquidambar styraciflua ‘Worplesdon’ | 1219 | Sequoia sempervirens |
| 518 | Liriodendron tulipifera | 1220 | Sequoiadendron giganteum |
| 519 | Liriodendron tulipifera ‘Snow Bird’ | 1221 | Sequoiadendron ‘Pendulum’ |
| 520 | Liriope | 1222 | Sesleria |
| 521 | Lithodora | 1223 | Sophora japonica ‘Gold Standard’ |
| 522 | Lobelia | 1224 | Sorbaria |
| 523 | Lonicera | 1225 | Sorbaronia ‘Likjormaja Liquorice’ |
| 524 | Lonicera nitida | 1226 | Sorbus |
| 525 | Lonicera periclymenum | 1227 | Sorbus alnifolia ‘Red Bird’ |
| 526 | Lupinus | 1228 | Sorbus ‘Amber Light’ |
| 527 | Luzula | 1229 | Sorbus aria |
| 528 | Lycium barbarum ‘Lubera Instant Success’ | 1230 | Sorbus aria ‘Lutescens’ |
| 529 | Lysimachia | 1231 | Sorbus aria ‘Majestica’ |
| 530 | Magnolia | 1232 | Sorbus arnoldiana ‘Golden Wonder’ |
| 531 | Magnolia ‘Aphrodite’ | 1233 | Sorbus arranensis |
| 532 | Magnolia ‘Black Tulip’ | 1234 | Sorbus aucuparia |
| 533 | Magnolia ‘Blue Opal’ | 1235 | Sorbus aucuparia ‘Aspleniifolia’ |
| 534 | Magnolia ‘Cleopatra’ | 1236 | Sorbus aucuparia ‘Beissneri’ |
| 535 | Magnolia ‘Daphne’ | 1237 | Sorbus aucuparia ‘Cardinal Royal’ |
| 536 | Magnolia ‘Daybreak’ | 1238 | Sorbus aucuparia ‘Croft Coral’ |
| 537 | Magnolia ‘Eskimo’ | 1239 | Sorbus aucuparia ‘Fingerprint’ |
| 538 | Magnolia ‘Fairy Blush’ | 1240 | Sorbus aucuparia ‘Sheerwater Seedling’ |
| 539 | Magnolia ‘Fairy Cream’ | 1241 | Sorbus aucuparia ‘Streetwise’ |
| 540 | Magnolia ‘Fairy White’ | 1242 | Sorbus ‘Autumn Spire’ |
| 541 | Magnolia ‘Felix Jury’ | 1243 | Sorbus bissetii ‘Pearls’ |
| 542 | Magnolia ‘Galaxy’ | 1244 | Sorbus ‘Cardinal Royal’ |
| 543 | Magnolia ‘Genie’ | 1245 | Sorbus carmesina ‘Emberglow’ |
| 544 | Magnolia ‘Golden Pond’ | 1246 | Sorbus cashmiriana |
| 545 | Magnolia grandiflora ‘Alta’ | 1247 | Sorbus ‘Chinese Lace’ |
| 546 | Magnolia grandiflora ‘Ferruginea’ | 1248 | Sorbus commixta ‘Embley’ |
| 547 | Magnolia grandiflora ‘Kay Parris’ | 1249 | Sorbus commixta ‘Olympic Flame’ |
| 548 | Magnolia ‘Heaven Scent’ | 1250 | Sorbus ‘Copper Kettle’ |
| 549 | Magnolia ‘Honey Tulip’ | 1251 | Sorbus discolor |
| 550 | Magnolia ‘Hot Flash’ | 1252 | Sorbus ‘Eastern Promise’ |
| 551 | Magnolia ‘Joli Pompom’ | 1253 | Sorbus ‘Ghose’ |
| 552 | Magnolia kobus | 1254 | Sorbus ‘Glendoick Spire’ |
| 553 | Magnolia ‘Livingstone’ | 1255 | Sorbus ‘Glendoick White Baby’ |
| 554 | Magnolia ‘March‐Till‐Frost’ | 1256 | Sorbus ‘Glowing Pink’ |
| 555 | Magnolia ‘Peachy’ | 1257 | Sorbus gonggashanica ‘Snow Balls’ |
| 556 | Magnolia ‘Red as Red’ | 1258 | Sorbus hemsleyi ‘John Bond’ |
| 557 | Magnolia ‘Satisfaction’ | 1259 | Sorbus hupehensis |
| 558 | Magnolia ‘Shirazz’ | 1260 | Sorbus hybrida ‘Gibbsii’ |
| 559 | Magnolia ‘Spectrum’ | 1261 | Sorbus intermedia |
| 560 | Magnolia ‘Sunsation’ | 1262 | Sorbus japonica |
| 561 | Magnolia ‘Susan’ | 1263 | Sorbus ‘John Mitchell’ |
| 562 | Magnolia ‘Watermelon’ | 1264 | Sorbus ‘Joseph Rock’ |
| 563 | Magnolia wilsonii ‘Eileen Baines’ | 1265 | Sorbus ‘Leonard Messel’ |
| 564 | Magnolia × brooklynensis ‘Yellow Bird’ | 1266 | Sorbus ‘Matthew Ridley’ |
| 565 | Mahonia | 1267 | Sorbus ‘Pink Ness’ |
| 566 | Malus | 1268 | Sorbus ‘Pink Pearl’ |
| 567 | Malus × purpurea ‘Crimson Cascade’ | 1269 | Sorbus pseudohupehensis ‘Pink Pagoda’ |
| 568 | Malus ‘Adam's Pearmain’ | 1270 | Sorbus pseudovilmorinii |
| 569 | Malus ‘Adirondack’ | 1271 | Sorbus ‘Ravensbill’ |
| 570 | Malus ‘Admiration’ | 1272 | Sorbus ‘Rose Queen’ |
| 571 | Malus ‘Angela’ | 1273 | Sorbus sargentiana |
| 572 | Malus ‘Annie Elizabeth’ | 1274 | Sorbus scalaris |
| 573 | Malus ‘Aros’ | 1275 | Sorbus splendens |
| 574 | Malus ‘Arthur Turner’ | 1276 | Sorbus ‘Sunshine’ |
| 575 | Malus ‘Ashmead's Kernel’ | 1277 | Sorbus thibetica ‘John Mitchell’ |
| 576 | Malus baccata | 1278 | Sorbus torminalis |
| 577 | Malus ‘Ballerina Flamenco’ | 1279 | Sorbus ulleungensis ‘Olympic Flame’ |
| 578 | Malus ‘Ballerina Samba’ | 1280 | Sorbus vilmorinii |
| 579 | Malus ‘Bardsey’ | 1281 | Sorbus vilmorinii ‘Pink Charm’ |
| 580 | Malus ‘Beauty of Bath’ | 1282 | Sorbus wardii |
| 581 | Malus ‘Black Dabinett’ | 1283 | Sorbus ‘Wisley Gold’ |
| 582 | Malus ‘Bladon Pippin’ | 1284 | Sorbus × thuringiaca ‘Fastigiata’ |
| 583 | Malus ‘Blenheim Orange’ | 1285 | Spiraea |
| 584 | Malus ‘Bloody Ploughman’ | 1286 | Stachys |
| 585 | Malus ‘Bountiful’ | 1287 | Stachyurus |
| 586 | Malus ‘Braeburn’ | 1288 | Stewartia pseudocamellia |
| 587 | Malus ‘Braeburn Mariri Red’ | 1289 | Stipa |
| 588 | Malus ‘Bramley 20’ | 1290 | Styrax japonicus ‘Fragrant Fountain’ |
| 589 | Malus ‘Bramley Original’ | 1291 | Styrax japonicus ‘June Snow’ |
| 590 | Malus ‘Bramley's Seedling’ | 1292 | Styrax japonicus ‘Pink Snowbell’ |
| 591 | Malus brevipes ‘Wedding Bouquet’ | 1293 | Symphoricarpus |
| 592 | Malus ‘Browns’ | 1294 | Symphytum |
| 593 | Malus ‘Butterball’ | 1295 | Syringa |
| 594 | Malus ‘Candymint’ | 1296 | Syringa ‘Pink Perfume’ |
| 595 | Malus ‘Cardinal’ | 1297 | Syringa vulgare ‘Beauty of Moscow’ |
| 596 | Malus ‘Charles Ross’ | 1298 | Syringa vulgare ‘Charles Joly’ |
| 597 | Malus ‘Chivers Delight’ | 1299 | Syringa vulgare ‘Katherine Havemeyer’ |
| 598 | Malus ‘Christmas P’ | 1300 | Syringa vulgare ‘Madame Lemoine’ |
| 599 | Malus ‘Christmas Pippin’ | 1301 | Syringa vulgare ‘Mrs Edward Harding’ |
| 600 | Malus ‘Cinderella’ | 1302 | Syringa vulgare ‘Primrose’ |
| 601 | Malus ‘Cobra’ | 1303 | Syringa vulgare ‘Sensation’ |
| 602 | Malus ‘Comtesse de Paris’ | 1304 | Syringa vulgare ‘Souvenir de Louis Spaeth’ |
| 603 | Malus ‘Coralburst’ | 1305 | Taxodium distichum |
| 604 | Malus ‘Core Blimey’ | 1306 | Taxodium distichum ‘Nutans’ |
| 605 | Malus ‘Cornish Aromatic’ | 1307 | Taxodium distichum ‘Shawnee Brave’ |
| 606 | Malus coronaria ‘Elk River’ | 1308 | Taxodium distichum var. imbricarium ‘Nutans’ |
| 607 | Malus ‘Coul Blush’ | 1309 | Taxus |
| 608 | Malus ‘Cox’ | 1310 | Taxus baccata |
| 609 | Malus ‘Cox Lavera’ | 1311 | Taxus baccata ‘Fastigiata Robusta’ |
| 610 | Malus ‘Cox Self Fertile’ | 1312 | Taxus baccata ‘Standishii’ |
| 611 | Malus ‘Cox's Orange Pippin’ | 1313 | Tellima |
| 612 | Malus ‘Dabinett’ | 1314 | Tetradium daniellii |
| 613 | Malus ‘Devonshire Quarrenden’ | 1315 | Thalictrum |
| 614 | Malus ‘Discovery’ | 1316 | Thuja |
| 615 | Malus ‘Discovery NFT’ | 1317 | Thuja plicata |
| 616 | Malus ‘Donald Wyman’ | 1318 | Thuja plicata ‘Fastigiata’ |
| 617 | Malus ‘Dr Campbells’ | 1319 | Thymus |
| 618 | Malus ‘Eden’ | 1320 | Tiarella |
| 619 | Malus ‘Egremont Russet’ | 1321 | Tilia |
| 620 | Malus ‘Ellison's Orange’ | 1322 | Tilia cordata |
| 621 | Malus ‘Evereste’ | 1323 | Tilia cordata ‘Corzam’ |
| 622 | Malus ‘Fiesta’ | 1324 | Tilia cordata ‘Greenspire’ |
| 623 | Malus florentina | 1325 | Tilia cordata ‘Streetwise’ |
| 624 | Malus floribunda | 1326 | Tilia cordata ‘Winter Orange’ |
| 625 | Malus ‘Fortune’ | 1327 | Tilia euchlora |
| 626 | Malus ‘Freja’ | 1328 | Tilia ‘Harold Hillier’ |
| 627 | Malus ‘Gala’ | 1329 | Tilia henryana |
| 628 | Malus ‘Galloway Pippin’ | 1330 | Tilia henryana ‘Arnolds Select’ |
| 629 | Malus ‘Gilly’ | 1331 | Tilia oliveri |
| 630 | Malus ‘Golden Delicious’ | 1332 | Tilia petolaris |
| 631 | Malus ‘Golden Gem’ | 1333 | Tilia platanoides |
| 632 | Malus ‘Golden Glory’ | 1334 | Tilia platanoides ‘Tiltstone Filigree’ |
| 633 | Malus ‘Golden Hornet’ | 1335 | Tilia platyphyllos |
| 634 | Malus ‘Gorgeous’ | 1336 | Tilia platyphyllos ‘Aurea’ |
| 635 | Malus ‘Granny Smith’ | 1337 | Tilia platyphyllos Princes Street’ |
| 636 | Malus ‘Greensleeves’ | 1338 | Tilia platyphyllos ‘Streetwise’ |
| 637 | Malus ‘Grenadier’ | 1339 | Tilia tomentosa ‘Brabant’ |
| 638 | Malus ‘Halloween’ | 1340 | Tilia × euchlora |
| 639 | Malus ‘Harry Baker’ | 1341 | Tilia × europaea ‘Golden Sunset’ |
| 640 | Malus ‘Harry M Jersey’ | 1342 | Tilia × europaea ‘Pallida’ |
| 641 | Malus ‘Hastings’ | 1343 | Tilia × europaea ‘Wratislaviensis’ |
| 642 | Malus ‘Herefordshire Russet’ | 1344 | Trachelospermum |
| 643 | Malus ‘Hidden Rose’ | 1345 | Trachycarpus fortunei |
| 644 | Malus ‘Honeycrisp’ | 1346 | Tradescantia |
| 645 | Malus ‘Howgate Wonder’ | 1347 | Tricyrtis |
| 646 | Malus hupehensis | 1348 | Trollius |
| 647 | Malus Indian ‘Magic’ | 1349 | Tsuga heterophylla |
| 648 | Malus ioensis ‘Fimbriata’ | 1350 | Ulex |
| 649 | Malus ioensis ‘Purpurea EVELYN’ | 1351 | Ulex europaeus |
| 650 | Malus ‘Irish Peach’ | 1352 | Ulmus |
| 651 | Malus ‘Isaac Newton’ | 1353 | Ulmus ‘Columnella’ |
| 652 | Malus ‘James Grieve’ | 1354 | Ulmus ‘Fiorente’ |
| 653 | Malus ‘Jelly King’ | 1355 | Ulmus glabra |
| 654 | Malus ‘John Downie’ | 1356 | Ulmus ‘New Horizon’ |
| 655 | Malus ‘Julia's Late Golden’ | 1357 | Ulmus ‘Rebona’ |
| 656 | Malus ‘Jumbo’ | 1358 | Ulmus ‘San Zenobi’ |
| 657 | Malus ‘Jupiter’ | 1359 | Ulmus ‘Wingham’ |
| 658 | Malus ‘Katy’ | 1360 | Ulmus × hollandica ‘Wredei’ |
| 659 | Malus ‘Keswick Codlin’ | 1361 | Uncinia |
| 660 | Malus ‘Kidd's Orange Red’ | 1362 | Vaccinium ‘Bluecrop’ |
| 661 | Malus ‘King of the Pippins’ | 1363 | Vaccinium ‘Chandler’ |
| 662 | Malus ‘King's Acre Pippin’ | 1364 | Vaccinium ‘Darrow’ |
| 663 | Malus ‘Kingston Black’ | 1365 | Vaccinium ‘Duke’ |
| 664 | Malus ‘Lady Henniker’ | 1366 | Vaccinium ‘Liberty’ |
| 665 | Malus ‘Lane's Prince Albert’ | 1367 | Vaccinium ‘Northland’ |
| 666 | Malus ‘Laura’ | 1368 | Vaccinium ‘Patriot’ |
| 667 | Malus ‘Laxton's Superb’ | 1369 | Vaccinium ‘Pink Lemonade’ |
| 668 | Malus ‘Limelight’ | 1370 | Vaccinium ‘Sunshine Blue’ |
| 669 | Malus ‘Little Pax’ | 1371 | Verbena |
| 670 | Malus ‘Lord Derby’ | 1372 | Veronica |
| 671 | Malus ‘Lord Lambourne’ | 1373 | Viburnum |
| 672 | Malus ‘Louisa’ | 1374 | Viburnum lantana |
| 673 | Malus ‘Major’ | 1375 | Viburnum opulus |
| 674 | Malus ‘Marble’ | 1376 | Viburnum opulus ‘Roseum’ |
| 675 | Malus ‘Melrose Belmonte’ | 1377 | Viburnum plicatum ‘Kilimanjaro’ |
| 676 | Malus ‘Meridian’ | 1378 | Vinca |
| 677 | Malus ‘Michelin’ | 1379 | Vitis ‘Bacchus’ |
| 678 | Malus ‘Mokum’ | 1380 | Vitis ‘Dornfelder’ |
| 679 | Malus ‘Newton Wonder’ | 1381 | Vitis ‘Lakemont’ |
| 680 | Malus ‘Orleans Reinette’ | 1382 | Vitis ‘Muscat Bleu’ |
| 681 | Malus ‘Paradice Gold’ | 1383 | Vitis ‘Phoenix’ |
| 682 | Malus ‘Peasgood's Nonsuch’ | 1384 | Vitis ‘Polo Muscat’ |
| 683 | Malus ‘Pink Glow’ | 1385 | Vitis ‘Regent’ |
| 684 | Malus ‘Pink Perfection’ | 1386 | Vitis ‘Strawberry’ |
| 685 | Malus ‘Pinot Prince SUPERNOVA’ | 1387 | Vitis ‘Suffolk Red’ |
| 686 | Malus ‘Pitmaston Pine Apple’ | 1388 | Weigela |
| 687 | Malus ‘Pixie’ | 1389 | Wisteria brachybotrys ‘Golden Dragon’ |
| 688 | Malus ‘Porters Perfection’ | 1390 | Wisteria brachybotrys ‘Kapiteyn Fugi’ |
| 689 | Malus ‘Prairie Fire’ | 1391 | Wisteria brachybotrys ‘Okayama’ |
| 690 | Malus ‘Prince William’ | 1392 | Wisteria brachybotrys ‘Shiro Beni’ |
| 691 | Malus ‘Professor Sprenger’ | 1393 | Wisteria ‘Burford’ |
| 692 | Malus ‘Queen Cox’ | 1394 | Wisteria floribunda ‘Black Dragon’ |
| 693 | Malus ‘Queen of the Realm’ | 1395 | Wisteria floribunda ‘Hon‐beni’ |
| 694 | Malus ‘Red Devil’ | 1396 | Wisteria sinensis |
| 695 | Malus ‘Red Falstaff’ | 1397 | Wisteria sinensis ‘Prolific’ |
| 696 | Malus ‘Red Foxwhelp’ | 1398 | × Cupressocyparis leylandii |
| 697 | Malus ‘Red Jonaprince’ | 1399 | Xanthocyparis nootkatensis ‘Pendula’ |
| 698 | Malus ‘Red Obelisk’ | 1400 | Yucca |
| 699 | Malus ‘Red Topaz’ | 1401 | Yucca filamentosa |
| 700 | Malus ‘Red Windsor’ | 1402 | Zelkova serrata ‘Green Vase’ |
| 701 | Malus ‘Reverend W. Wilks’ | 1403 | Zelkova serrata ‘Kiwi Sunset’ |
| 702 | Malus ‘Ribston Pippin’ |
APPENDIX D. Water used for irrigation
D.1.
All mains water used meets the UK standard Water Supply (Water quality) regulation 2016 and the WHO/EU potable water standards, (Drinking water Directive (98/83/EC and the revised Drinking Water Directive 2020/2184)) which includes a total freedom from both human and plant pathogens (Article 2‐(7)). All mains water conducting pipework fully complies with the UK Water Supply (Water Fittings) regulations of 1999 and the amendments of 2019. Irrigation water used is not stored in any open tanks where air borne contamination could take place and is entirely isolated from any outside exposure (Dossier Sections 1.1 and 1.2).
Bore hole water supply: in some cases, where the underlying geology permits, nurseries can draw water directly from bore holes drilled into underground aquafers. The water that fills these aquafers is naturally filtered through the layers of rock (e.g. limestone) over long periods of time, many millennia in some cases. The water from such supplies is generally of such high quality that it is fit for human consumption with little to no further processing and is often bottled and sold as mineral water (Dossier Sections 1.1 and 1.2).
Rainwater or freshwater watercourse supply: some nurseries contributing to this application for both environmental and efficiency reasons use a combination of rain capture systems or abstract directly from available watercourses. All water is passed through a sand filtration system to remove contaminants and is contained in storage tanks prior to use. One nursery that operates this approach is currently in the process of installing additional nanobubble technology to treat the water (Dossier Sections 1.1 and 1.2).
APPENDIX E. List of pests that can potentially cause an effect not further assessed
E.1.
TABLE E.1 List of potential pests not further assessed.
| N | Pest name | EPPO code | Group | Pest present in the UK | Present in the EU | Betula confirmed as a host (reference) | Pest can be associated with the commodity | Impact | Justification for inclusion in this list |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Acremonium apii | ACREAP | Fungi | Yes | Limited | Betula pendula (Farr & Rossman, 2024) | Uncertain | No data | Uncertainty on impact and association with the commodity |
APPENDIX F. Excel file with the pest list of Betula pendula and B. pubescens
F.1.
Appendix F can be found in the online version of this output (in the ‘Supporting Information section’): https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2024.9051#support‐informationsection.
EFSA PLH Panel (EFSA Panel on Plant Health) , Civera, A. V. , Baptista, P. , Berlin, A. , Chatzivassiliou, E. , Cubero, J. , Cunniffe, N. , de la Peña, E. , Desneux, N. , Di Serio, F. , Filipiak, A. , Hasiów‐Jaroszewska, B. , Jactel, H. , Landa, B. B. , Maistrello, L. , Makowski, D. , Milonas, P. , Papadopulos, N. T. , Potting, R. , … Gonthier, P. (2024). Commodity risk assessment of Betula pendula and Betula pubescens plants from the UK . EFSA Journal, 22(11), e9051. 10.2903/j.efsa.2024.9051
Adopted: 26 september 2024
Notes
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants, amending Regulations (EU) 228/2013, (EU) 652/2014 and (EU) 1143/2014 of the European Parliament and of the Council and repealing Council Directives 69/464/EEC, 74/647/EEC, 93/85/EEC, 98/57/EC, 2000/29/EC, 2006/91/EC and 2007/33/EC. OJ L 317, 23.11.2016, pp. 4–104.
Commission Implementing Regulation (EU) 2018/2019 of 18 December 2018 establishing a provisional list of high risk plants, plant products or other objects, within the meaning of Article 42 of Regulation (EU) 2016/2031 and a list of plants for which phytosanitary certificates are not required for introduction into the Union, within the meaning of Article 73 of that Regulation C/2018/8877. OJ L 323, 19.12.2018, pp. 10–15.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, pp. 1–24.
Commission Implementing Regulation (EU) 2019/2072 of 28 November 2019 establishing uniform conditions for the implementation of Regulation (EU) 2016/2031 of the European Parliament and the Council, as regards protective measures against pests of plants, and repealing Commission Regulation (EC) No 690/2008 and amending Commission Implementing Regulation (EU) 2018/2019. OJ L 319, 10.12.2019, p. 1–279.
In accordance with the Agreement on the withdrawal of the United Kingdom of Great Britain and Northern Ireland from the European Union and the European Atomic Energy Community, and in particular Article 5(4) of the Windsor Framework in conjunction with Annex 2 to that Framework, for the purposes of this Opinion, references to the United Kingdom do not include Northern Ireland.
Plant Health (Amendment etc.) (EU Exit) Regulations 2020 of 14 December 2020, No. 1482, 80 pp. https://www.legislation.gov.uk/uksi/2020/1482/contents/made.
Plant Health (Phytosanitary Conditions) (Amendment) (EU Exit) Regulations 2020, No. 1527, 276 pp. https://www.legislation.gov.uk/uksi/2020/1527/contents/made.
REFERENCES
- Anonymous . (1960). Index of plant diseases in the United States. USDA, Agricultrure Handbook 165, 531 pp. https://www.govinfo.gov/content/pkg/GOVPUB‐A‐PURL‐gpo20004/pdf/GOVPUB‐A‐PURL‐gpo20004.pdf
- Atkinson, T. H. (2024). Bark and Ambrosia Beetles of the Americas. https://www.barkbeetles.info/index.php (accessed: 2024‐03‐21).
- Barringer, L. , & Ciafré, C. M. (2020). Worldwide feeding host plants of spotted lanternfly, with significant additions from North America. Environmental Entomology, 49(5), 999–1011. [DOI] [PubMed] [Google Scholar]
- Ciesla, W. M. , & Kruse, J. J. (2009). Large Aspen Tortrix. USDA Forest Service. Forest Insect & Disease Leaflet, 139, 8. [Google Scholar]
- Clark, S. M. , LeDoux, D. G. , Seeno, T. N. , Riley, E. G. , Gilbert, A. J. , & Sullivan, J. M. (2004). Host plants of leaf beetle species occurring in the United States and Canada (Coleoptera: Megalopodidae, Orsodacnidae, Chrysomelidae, excluding Bruchinae). Coleopterists society, special Publication, 2, 615. [Google Scholar]
- den Nijs, L. J. M. F. , Brinkman, H. , & van der Sommen, A. T. C. (2004). A Dutch contribution to knowledge on phytosanitary risk and host status of various crops for Meloidogyne chitwoodi Golden et al., 1980 and M. fallax Karssen, 1996: An overview. Nematology, 6, 303–312. 10.1163/1568541042360492 [DOI] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) . (2018). Guidance on quantitative pest risk assessment. EFSA Journal, 16(8), 5350. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) . (2019a). Guidance on commodity risk assessment for the evaluation of high risk plants dossiers. EFSA Journal, 17(4), 5668. 10.2903/j.efsa.2019.5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard, C. , Dehnen‐Schmutz, K. , Di Serio, F. , Gonthier, P. , Jacques, M.‐A. , Jaques Miret, J. A. , Justesen, A. F. , MacLeod, A. , Magnusson, C. S. , Navas‐Cortes, J. A. , Parnell, S. , Potting, R. , Reignault, P. L. , Thulke, H.‐H. , Van der Werf, W. , Vicent Civera, A. , Yuen, J. , Zappala, L. , … Milonas, P. (2019b). Scientific opinion on the pest categorisation of non‐EU Acleris spp. EFSA Journal, 17(10), 5856. 10.2903/j.efsa.2019.5856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard, C. , Baptista, P. , Chatzivassiliou, E. , Di Serio, F. , Jaques Miret, J. A. , Justesen, A. F. , MacLeod, A. , Magnusson, C. S. , Milonas, P. , Navas‐Cortes, J. A. , Parnell, S. , Potting, R. , Reignault, P. L. , Stefani, E. , Thulke, H.‐H. , Van der Werf, W. , Vicent Civera, A. , Yuen, J. , … Gonthier, P. (2023a). Scientific opinion on the commodity risk assessment of Acer platanoides plants from the UK. EFSA Journal, 21(7), 8073 10.2903/j.efsa.2023.8073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard, C. , Baptista, P. , Chatzivassiliou, E. , Di Serio, F. , Jaques Miret, J. A. , Justesen, A. F. , MacLeod, A. , Magnusson, C. S. , Milonas, P. , Navas‐Cortes, J. A. , Parnell, S. , Potting, R. , Reignault, P. L. , Stefani, E. , Thulke, H.‐H. , Van der Werf, W. , Vicent Civera, A. , Yuen, J. , … Gonthier, P. (2023b). Scientific Opinion on the commodity risk assessment of Acer campestre plants from the UK. EFSA Journal, 21(7), 8071. 10.2903/j.efsa.2023.8071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard, C. , Baptista, P. , Chatzivassiliou, E. , Di Serio, F. , Jaques Miret, J. A. , Justesen, A. F. , MacLeod, A. , Magnusson, C. S. , Milonas, P. , Navas‐Cortes, J. A. , Parnell, S. , Potting, R. , Reignault, P. L. , Stefani, E. , Thulke, H.‐H. , Van der Werf, W. , Vicent Civera, A. V. , Yuen, J. , … Gonthier, P. (2024). Commodity risk assessment of Corylus avellana plants from the UK. EFSA Journal, 22(1), e8495 10.2903/j.efsa.2024.8495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee . (2018). Scientific Opinion on the principles and methods behind EFSA's guidance on uncertainty analysis in scientific assessment. EFSA Journal, 16(1), 5122. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskalen, A. , Stouthamer, R. , Lynch, S. C. , Rugman‐Jones, P. F. , Twizeyimana, M. , Gonzalez, A. , & Thibault, T. (2013). Host range of Fusarium dieback and its ambrosia beetle (Coleoptera: Scolytinae) vector in southern California. Plant Disease, 97(7), 938–951. [DOI] [PubMed] [Google Scholar]
- EUROPHYT (European Union Notification System for Plant Health Interceptions) . (2024). https://food.ec.europa.eu/plants/plant‐health‐and‐biosecurity/europhyt_en (accessed 2024‐02‐09).
- FAO (Food and Agriculture Organization of the United Nations) . (2019). ISPM (international standards for phytosanitary measures) No 36. Integrated measures for plants for planting. FAO. https://www.ippc.int/en/publications/636 [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) . (2024a). ISPM (International standards for phytosanitary measures) No 4. Requirements for the establishment of pest free areas. FAO. https://www.ippc.int/en/publications/614/ [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) . (2024b). ISPM (international standards for phytosanitary measures) No. 5. Glossary of phytosanitary terms. FAO. https://www.ippc.int/en/publications/622/ [Google Scholar]
- Farr, D. F. , & Rossman, A. Y. (2024). Fungal Databases, U.S. National Fungus Collections, ARS, USDA. https://fungi.ars.usda.gov/ (accessed 2024‐04‐18).
- Ferguson, D. C. (1975). Host records for Lepidoptera reared in eastern North America. USDA Technical Bulletin, 1521, 1–49. [Google Scholar]
- Fleming, W. E. (1972). Biology of the Japanese beetle. Technical Bulletin, Agricultural Research Service, USDA no 1449, 129 pp.
- Ginns, J. H. (1986). Compendium of plant disease and decay fungi in Canada 1960–1980. Research branch. Canada. Agriculture Publication 1813, 416 pp. https://publications.gc.ca/collections/collection_2021/aac‐aafc/A43‐1813‐1986‐eng.pdf
- Granmo, A. , Læssøe, T. , & Schumacher, T. (1999). The genus sl (Xylariaceae) in Norden. Sommerfeltia, 27(1), 100. [Google Scholar]
- Hayat, U. (2022). City longhorn beetle (Aeolesthes sarta): A review of the species, its distribution, ecology, damage, prevention and control. Journal of Forest Science, 68(6), 199–212. [Google Scholar]
- Kottek, M. , Grieser, J. , Beck, C. , Rudolf, B. , & Rubel, F. (2006). World map of Köppen‐Geiger climate classification updated. Meteorologische Zeitschrift, 15, 259–263. [Google Scholar]
- Lu, W. , & Wang, Q. I. A. O. (2005). Systematics of the New Zealand longicorn beetle genus Oemona Newman with discussion of the taxonomic position of the Australian species, O. simplex White (Coleoptera: Cerambycidae: Cerambycinae). Zootaxa, 971(1), 31. [Google Scholar]
- Peng, Y. , Buranapanichpan, A. , & Kamata, N. (2022). Succession of Ambrosia beetles colonizing the logs of fallen alder and birch trees. Insects, 13(3), 223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamour, F. S. (1999). Progress in the development of borer‐resistant white‐barked birches. Journal of Arboriculture, 25(3), 151–162. [Google Scholar]
- Shrewsbury, P. M. , Harding, N. M. , Rojas, M. S. , & Gill, S. (2013). Japanese maple scale: Woody ornamental host plants. UMD Extension Publication EBR‐18 2013. https://extension.umd.edu/sites/extension.umd.edu/files/publications/Japanese%20Maple%20Scale%20%282%29.pdf
- Siddiqui, I. A. , Sher, S. A. , & French, A. M. (1973). Distribution of plant parasitic nematodes in California. State of California Department of Food and Agriculture, Division of Plant Industry. 324 pp.
- Sjöman, H. , Östberg, J. , & Nilsson, J. (2014). Review of host trees for the wood‐boring pests Anoplophora glabripennis and Anoplophora chinensis: An urban forest perspective. Arboriculture & Urban Forestry, 40(3), 143–164. [Google Scholar]
- Stigter, H. , Geraedts, W. H. J. M. , & Spijkers, H. C. P. (1997). Thaumetopoea processionea in The Netherlands: Present status and management perspectives (Lepidoptera: Notodontidae). Proceedings of the section experimental and applied entomology of The Netherlands entomological society (N.E.V.), 3–16.
- Takaro, T. (2013). Determinants of tree susceptibility to attack by the red alder bark beetle, Alniphagus aspericollis (LeConte) (Coleoptera: Scolytidae) (Thesis). 23 pp.
- TRACES‐NT . (2024). TRAde Control and Expert System. https://webgate.ec.europa.eu/tracesnt (accessed 2024‐02‐09).
- USDA (U.S. Department of Agriculture) . (2024). Nematode Collection Database, Xiphinema rivesi . https://nematode.ars.usda.gov/?page=1622 (accessed 2024‐03‐21).
- Vlasak, J. , & Vlasakova, K. (2002). Records of Cerambycidae (Coleoptera) in Massachusetts with notes on larval hosts. The Coleopterists Bulletin, 56, 203–219. [Google Scholar]
- Webber, J. F. , Mullett, M. , & Brasier, C. M. (2010). Dieback and mortality of plantation Japanese larch (Larix kaempferi) associated with infection by Phytophthora ramorum . New Disease Reports, 22, 19. [Google Scholar]
- Wood, S. L. , & Bright, D. E. (1992). A catalog of Scolytidae and Platypodidae (Coleoptera). Part 2: Taxonomic index. Great Basin Naturalist Memoirs, 13, 1241–1348. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Excel file with the pest list of Betula pendula and B. pubescens


