Fig. 3.
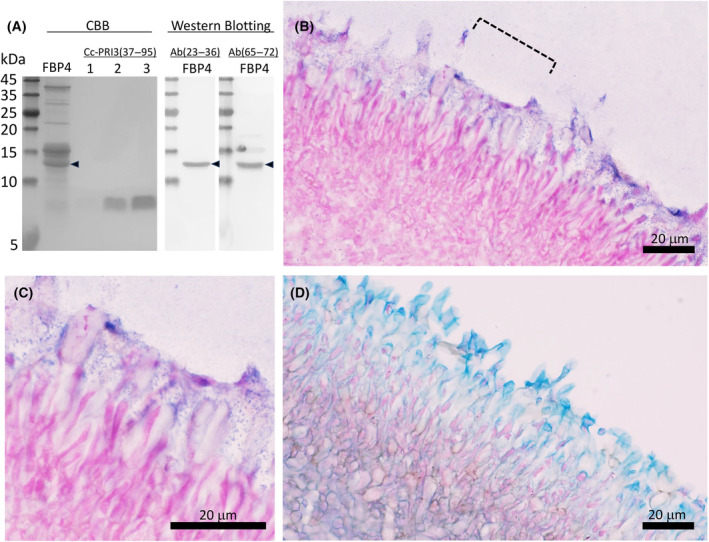
Western blotting and immunohistochemical analysis of native Cc‐PRI3 protein in fruiting body. (A) Lysate of the pileus of fruiting body of about 4‐cm height (FBP4) was centrifuged, and then the supernatant was precipitated stepwise by 40% ammonium sulfate and 80% ammonium sulfate. The proteins precipitated by 80% ammonium sulfate were heat‐treated in sample buffer containing 5 mm dithiothreitol and resolved by 18% SDS/PAGE (lanes: FBP4), transferred onto a membrane, and then detected using rabbit antiserum raised against a synthetic peptide representing residues 23–36 [Ab(23–36)] or residues 65–72 [Ab(65–72)] of the Cc‐PRI3 protein. Native Cc‐PRI3 (arrowhead) was detected as a band of 13 kDa using Coomassie Brilliant Blue (CBB) staining and both antisera (n = 2). The precipitates obtained using 40% ammonium sulfate showed no immunoreactive band (data not shown). Recombinant protein Cc‐PRI3(37–95) (lane 1, 0.1 μg; lane 2, 0.5 μg; lane 3, 1.0 μg) prepared in this study was used as a reference, and was detected as a band of 8.5 kDa by CBB staining (n = 2). (B) Immunohistochemical analysis using a thin section of the pileus of fruiting body of about 4‐cm height and the antiserum Ab(23–36) raised against the synthetic peptide representing residues 23–36 of Cc‐PRI3. Immunostaining with nitro blue tetrazolium and 5‐bromo‐4‐chrolo‐3‐indoyl phosphate (blue) and cell staining with Nuclear Fast Red solution (red) are shown (n = 3, representative images shown). Scale bar: 20 μm. The area indicated by the broken line is enlarged in panel C. Immunostaining was observed with a dot‐like pattern. Scale bar: 20 μm. (D) Thin section of the pileus of fruiting body of about 4‐cm height stained with Alcian blue solution (blue), which is specific for acidic polysaccharides, and with Nuclear Fast Red solution (red) (n = 2, representative images shown). Scale bar: 20 μm.
