Fig. 7.
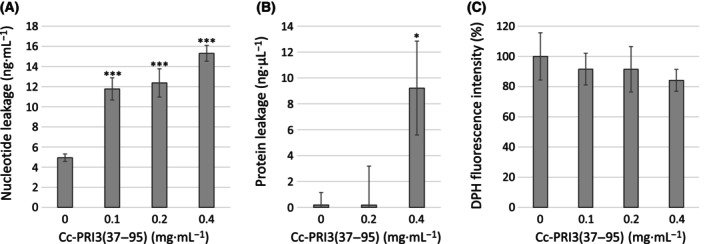
Assessment of membrane integrity of A. nidulans hyphae. (A) A. nidulans hyphae suspended in PBS were treated with recombinant Cc‐PRI3(37–95) (0, 0.1, 0.2, 0.4 mg·mL−1) at 30 °C for 5 h, and centrifuged. Supernatants were analyzed to determine concentrations of nucleotides leaked from hyphae using a fluorescent dye that binds selectively to double‐stranded DNAs over other nucleic acids. Each experiment was performed in triplicate, and all data are displayed as mean values ± SD. Statistically significant differences from control were determined using Student's t‐test and indicated as ***P < 0.001. (B) Similarly to (A), A. nidulans hyphae suspended in PBS were treated with the recombinant protein (0, 0.2, 0.4 mg·mL−1) at 30 °C for 5 h, and centrifuged. Supernatants were analyzed to determine concentrations of proteins leaked from the hyphae, using a fluorescent dye that binds selectively to proteins. Each experiment was performed in triplicate, and all data are displayed as mean values ± SD. Statistically significant difference from control was determined using Student's t‐test and indicated as *P < 0.05. (C) A. nidulans hyphae in liquid medium (2% glucose and 0.1% dry bouillon; w/v) were treated with the recombinant protein (0, 0.1, 0.2, 0.4 mg·mL−1) at 30 °C overnight, washed with PBS, and then incubated with 0.6 mm 1,6‐diphenyl‐1,3,5‐hexatriene (DPH). After washing with PBS, relative fluorescence intensity of DPH in the hyphae was assessed. Each experiment was performed in triplicate, and all data are displayed as mean values ± SD. The DPH fluorescence intensity decreased, as the concentration of the recombinant protein increased, although statistically significant differences (P < 0.05) from control were not observed using Student's t‐test.
