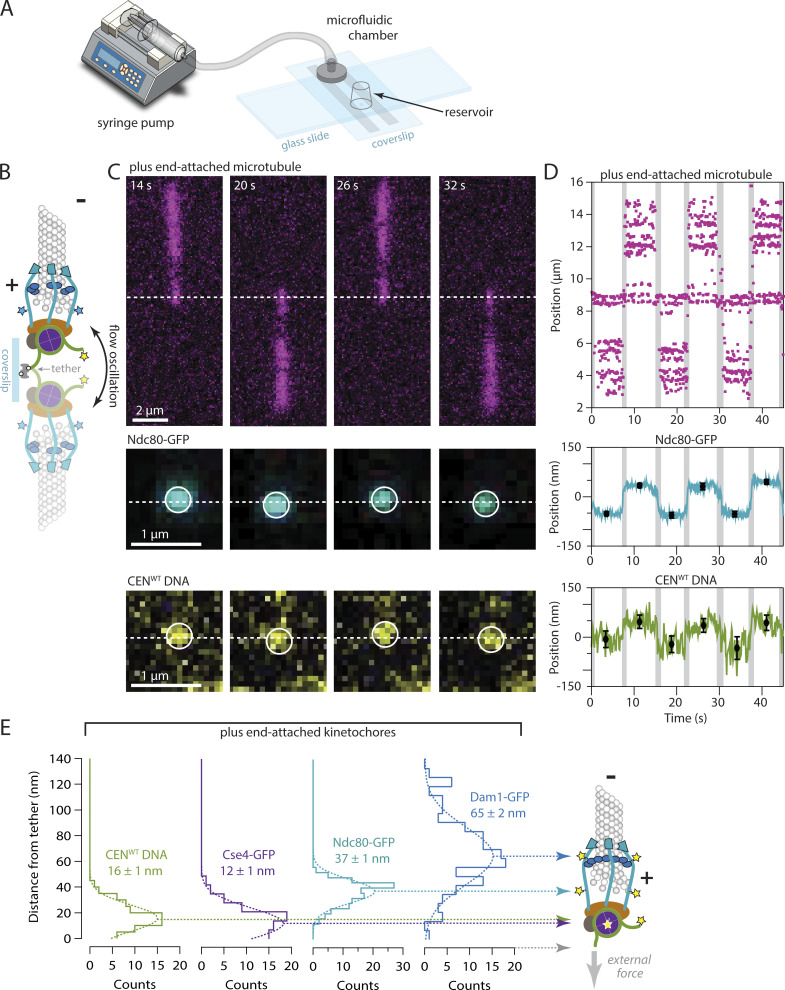
Figure 3.
Plus-end-attached kinetochores are well organized along the microtubule axis. (A) Kinetochores were assembled in a microfluidic device and then allowed to capture microtubules. A syringe pump enabled imaging of the kinetochores and their captured microtubules while the buffer flowed gently through the assembly chamber. (B) Schematic of a surface-assembled kinetochore attached to the tip of a microtubule. Oscillating the direction of flow caused the kinetochore and its captured microtubule to flip back and forth, reorienting by 180° around the biotin-avidin tether with each reversal of the flow. (C) Time-lapse image series showing flow-induced reorientation of a microtubule (magenta) attached by its end to a surface-assembled kinetochore. Both the Ndc80-GFP kinetochore marker (cyan) and the Atto565 label on the wild type centromeric DNA (yellow, CENWT) oscillated with the direction of buffer flow. Horizontal dashed lines indicate approximate positions of the DNA tether point on the coverslip. (D) Example records of position versus time for an Ndc80-GFP spot and the corresponding Atto565-labeled centromeric DNA obtained by tracking the individual spots with subpixel accuracy. Displacements of each spot from the tether point were estimated by averaging during the intervals when the microtubule orientation was steady. The position of the biotin–avidin tether point was inferred as the midpoint between tracked positions before and after each flow reversal. Black symbols represent mean ± SD from N = 60 tracked positions during each interval. Positions recorded during the reorientation of the microtubule were omitted from the averaging and are indicated here by gray shading. Additional records are shown in Fig. S4. (E) Distributions of displacement for the indicated fluorescent kinetochore components (from N = 67–128 intervals), fit with single Gaussian functions. The mean ± SEM for each Gaussian is indicated. Displacements for Cse4-GFP, a component of the centromeric nucleosome, are similar to the centromeric DNA (CENWT), as expected. The larger displacements for outer microtubule-binding components, Ndc80-GFP and Dam1-GFP, are consistent with the in vivo arrangement (Joglekar et al., 2009; Cieslinski et al., 2023).