Abstract
Objective
To examine disease and target engagement biomarkers in the RISE-SSc trial of riociguat in early diffuse cutaneous systemic sclerosis and their potential to predict the response to treatment.
Methods
Patients were randomized to riociguat (n = 60) or placebo (n = 61) for 52 weeks. Skin biopsies and plasma/serum samples were obtained at baseline and week 14. Plasma cyclic guanosine monophosphate (cGMP) was assessed using radio-immunoassay. α-Smooth muscle actin (αSMA) and skin thickness were determined by immunohistochemistry, mRNA markers of fibrosis by qRT-PCR in skin biopsies, and serum CXC motif chemokine ligand 4 (CXCL-4) and soluble platelet endothelial cell adhesion molecule-1 (sPECAM-1) by enzyme-linked immunosorbent assay.
Results
By week 14, cGMP increased by 94 (78)% with riociguat and 10 (39)% with placebo (P < 0.001, riociguat vs placebo). Serum sPECAM-1 and CXCL-4 decreased with riociguat vs placebo (P = 0.004 and P = 0.008, respectively). There were no differences in skin collagen markers between the two groups. Higher baseline serum sPECAM-1 or the detection of αSMA-positive cells in baseline skin biopsies was associated with a larger reduction of modified Rodnan skin score from baseline at week 52 with riociguat vs placebo (interaction P-values 0.004 and 0.02, respectively).
Conclusion
Plasma cGMP increased with riociguat, suggesting engagement with the nitric oxide–soluble guanylate cyclase–cGMP pathway. Riociguat was associated with a significant reduction in sPECAM-1 (an angiogenic biomarker) vs placebo. Elevated sPECAM-1 and the presence of αSMA-positive skin cells may help to identify patients who could benefit from riociguat in terms of skin fibrosis.
Trial registration
Clinicaltrials.gov, NCT02283762.
Keywords: biomarkers, diffuse cutaneous systemic sclerosis, riociguat, soluble guanylate cyclase stimulators
Rheumatology key messages.
Lower baseline serum PECAM-1 or absent αSMA-positive skin cells predicted greater mRSS decline with placebo.
Higher serum PECAM-1 or αSMA-positive cells predicted greater mRSS reductions with riociguat vs placebo.
These markers may identify progressors in early disease and patients who could benefit from riociguat.
Introduction
Systemic sclerosis (SSc) is a severe and debilitating autoimmune connective tissue disease. It is characterized by fibrosis, inflammation, microvascular injury and systemic organ manifestations including pulmonary arterial hypertension (PAH), interstitial lung disease, renal dysfunction and failure, diffuse gastrointestinal disease, and myocardial involvement [1–4]. The nitric oxide (NO)–soluble guanylate cyclase (sGC)–cyclic guanosine monophosphate (cGMP) pathway plays an important role in tissue homeostasis through various mechanisms including antifibrotic and anti-inflammatory effects [5, 6]. In preclinical in vitro and in vivo studies, the soluble guanylate cyclase stimulator riociguat exhibited anti-inflammatory, antifibrotic and antiproliferative effects mediated partly by the attenuation of TGF-β signalling [5–7]. The phase 3 PATENT trial of riociguat in PAH [8, 9] included a subgroup with PAH associated with SSc, in whom riociguat prevented the decline in functional capacity and was well tolerated [10]. In addition, riociguat improved digital blood flow in some patients with Raynaud’s phenomenon in a single-dose pilot study [11]. These observations suggested that riociguat may reduce tissue fibrosis in SSc, and led to the investigation of riociguat in the phase 2b RIociguat Safety and Efficacy in patients with early diffuse cutaneous Systemic Sclerosis (RISE-SSc) study [12]. Treatment with riociguat for 52 weeks did not significantly improve the primary end point (modified Rodnan skin score [mRSS]) vs placebo; however, a numerical decrease in mRSS was seen with riociguat (P = 0.08 vs placebo) and analyses of secondary and exploratory endpoints showed potential efficacy [12].
Prognostic biomarkers help to identify patients who are at high risk for certain disease outcomes, such as organ involvement or death in patients with diffuse cutaneous systemic sclerosis (dcSSc), irrespective of treatment. Predictive biomarkers allow physicians to predict response to treatment [13]. Both types of biomarkers may help to inform clinical decision-making and efforts have been made to identify predictive parameters for disease progression in dcSSc [14–16]. Target engagement biomarkers confirm delivery of the drug and indicate that it is acting on its target. This report describes the pre-specified exploratory biomarker analysis from RISE-SSc. The objectives were to examine the effects of riociguat on its target pharmacological pathway, to investigate the prognostic value of biomarkers in the placebo group (who did not receive targeted treatments for SSc other than rescue therapy at investigator discretion from week 26), and to investigate whether biomarkers could predict the effects of riociguat on skin fibrosis, measures of disease activity and progression of lung disease.
Methods
Study design
RISE-SSc (Clinicaltrials.gov NCT02283762) was a randomized, double-blind, placebo-controlled, phase 2b study of riociguat in patients fulfilling American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria for SSc [17], with dcSSc according to LeRoy and Medsager [18] and mRSS of 10–22 units (Supplementary Fig. S1, available at Rheumatology online) [12]. Patients were randomized 1:1 to receive riociguat or placebo up to 2.5 mg—maximum three times daily. From week 26, rescue therapy was permitted at the investigator’s discretion. The primary end point was the change in mRSS from baseline to week 52 with riociguat vs placebo [12].
Selection of biomarkers
Variable selection was performed applying the stability selection approach. Only markers that were selected in at least 20% (of 1000 repetitions) were further assessed; markers that did not show prognostic or predictive potential were excluded. The biomarkers selected are summarized in Table 1.
Table 1.
Summary of biomarkers evaluated
| Biomarker | Method |
|---|---|
| Markers of NO–sGC–cGMP system engagement/activity | |
| Plasma cGMP | RIA |
| Plasma ADMA and SDMA | HPLC–MS |
| p-ERK, p-VASP | IHC, skin biopsy |
| Thrombospondin-1 | RT-qPCR, skin biopsy |
| Inflammatory markers | |
| Serum hsCRP | ITA |
| Serum sE-selectin, CXCL-4, sPECAM | ELISA |
| Components of extracellular matrix | |
| Collagen 1A1, collagen 1A2, collagen 3A1, fibronectin, cartilage oligomeric matrix protein, | RT-qPCR, skin biopsy |
| Autoantibodies | |
| Anti-Scl-70 | Multiplex bead-based fluorescence immunoassay |
| Anti-RNA polymerase III | Semi-quantitative ELISA |
| αSMA | IHC, skin biopsy |
| Skin thickness | Histology/light microscopy, skin biopsy |
ADMA: asymmetric dimethylarginine; αSMA: α-smooth muscle actin; anti-scl-70: anti-topoisomerase I; cGMP: cyclic guanosine monophosphate; CXCL-4: CXC motif chemokine ligand 4; IHC: immunohistochemistry; ITA: immunoturbidimetry assay; NO: nitric oxide; p-ERK: phosphorylated extracellular signal-related kinase; p-VASP: phosphorylated vasodilator-stimulated phosphoprotein; RIA, radio-immunoassay; RT-qPCR: reverse transcription–quantitative real-time PCR; SDMA: symmetric dimethylarginine; sE-selectin: soluble E-selectin; sGC: soluble guanylate cyclase; sPECAM: soluble platelet endothelial cell adhesion molecule-1.
cGMP was selected as a marker of activation of the NO–sGC–cGMP pathway by riociguat [7]. Asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA) are endogenous inhibitors of NO [19], and ADMA levels are elevated in diffuse SSc [20]. Extracellular signal-related kinase (ERK) has been implicated in tissue fibrosis [21] and contractile activity in scleroderma fibroblasts [22], and TGF-β stimulates phosphorylation of ERK in dermal fibroblasts [21]. Vasodilator-stimulated phosphoprotein (VASP) is present in vascular smooth muscle cells, endothelial cells, and fibroblasts and is a substrate for cGMP-dependent protein kinases [23]. sGC stimulators have been shown to increase phosphorylation of VASP [23]. ADMA, SDMA, phosphorylated ERK (p-ERK), and phosphorylated VASP (p-VASP) were assessed as indicators of NO–sGC–cGMP pathway activation and TGF-β signalling. High-sensitivity C-reactive protein (hsCRP), soluble E-selectin (sE-selectin), soluble platelet endothelial cell adhesion molecule-1 (sPECAM-1, also referred to as CD31) and CXC motif chemokine ligand 4 (CXCL-4) are inflammatory markers that are elevated in patients with SSc and are associated with increased disease activity or progression [16, 24–27]. Collagen 1A1, collagen 1A2, collagen 3A1, fibronectin and cartilage oligomeric protein are components of extracellular matrix [3, 28]. Thrombospondin-1 is a mediator of TGF-β-mediated cell contractility in SSc [29]. Anti-Scl-70 (anti-topoisomerase) and anti-RNA polymerase III autoantibodies are included in the diagnostic criteria for SSc [17] and are associated with internal organ involvement and progressive skin disease [14–16, 24–26]. α-Smooth muscle actin (αSMA) is a marker of fibroblast cell proliferation, myofibroblast deposition and contractile force generation [30]. Myofibroblasts detected by αSMA immunofluorescence are present in fibrotic skin samples from patients with scleroderma but not in healthy skin or atrophic dcSSc skin [31]. Skin thickness was assessed as this is a characteristic feature of early dcSSc [1, 2].
Biopsy techniques and biomarker analyses
Skin biopsies and plasma or serum samples were obtained for biomarker assessment on day 0 and week 14. Techniques for specimen collection and biomarker measurement and interpretation are provided in the Supplementary Data S1, available at Rheumatology online (pp. 1–5). Anti-Scl-70 antibodies were assessed semi-quantitatively using a multiplex bead-based fluorescence immunoassay (FIDIS Connective 10, Theradiag, Croissy Beaubourg, France). Anti-RNA polymerase III antibodies were assessed with a semi-quantitative enzyme-linked immunosorbent assay (QUANTA Lite, INOVA Diagnostics, San Diego, CA, USA). Details of both antibody tests are provided in the Supplementary Data S1, available at Rheumatology online (p. 5). Staining for αSMA to detect myofibroblasts in skin biopsies has been used in other studies in SSc [32–34].
Statistical analysis
All biomarkers and their absolute changes from baseline were summarized descriptively by assigned treatment group and visit.
Analyses were performed using SAS System v9.2 or later (SAS Institute, Cary, NC, USA) and R software v3.1.0 or later (R Foundation for Statistical Computing, Vienna, Austria). The analysis was conducted on the intention-to-treat population. As the primary end point of RISE-SSc did not reach the predefined P < 0.05 level, all P-values reported here should be considered nominal. P-values were not adjusted for multiplicity due to the exploratory nature of the analyses, do not imply statistical significance and are for information only. For this report, Spearman’s correlation <0.3 between biomarkers or between biomarkers and endpoints is not generally shown because it would be of little scientific or clinical interest. Further details of statistical methods are described in the Supplementary Data S1, available at Rheumatology online (pp. 5–8).
Ethics statement
The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice. The study was approved by the University of Michigan Institutional Review Board and by the ethics committee or institutional review board of each participating site. All patients provided written informed consent.
Results
Study population and baseline biomarkers
The primary results of the double-blind phase of RISE-SSc have been published [12]. In total, 121 patients were randomized (riociguat, n = 60; placebo, n = 61). Mean (s.d.) mRSS was 16.8 (3.7) overall at baseline (riociguat, 16.9 [3.4]; placebo, 16.7 [4.1]) and 14.6 (6.6) and 15.7 (10.5) in the riociguat and placebo groups, respectively, at week 52. Baseline levels of biomarkers were generally similar between the two groups (Table 2). Three values considered outliers were removed from the data (i.e. set to ‘missing’) for all analyses of the marker in question because they would have disproportionately affected the results. Results for hsCRP were removed for two patients because of values at week 14 that were >14-fold and >290-fold greater than baseline (baseline, 1.8 and 0.3 mg/l; week 14, 25.9 and 88.3 mg/l, respectively). Results for CXCL-4 were removed for one patient because the level at week 14 was 3.8 mg/l, which was not considered credible. In addition, immunohistochemistry markers (baseline and week 14), ADMA (week 14) and SDMA (week 14) were excluded due to withdrawal of informed consent by one patient.
Table 2.
Key baseline biomarker levels
| Biomarker, mean (s.d.) [median; 10‒90 percentile] | Placebo group (n = 61) | Riociguat group (n = 60) |
|---|---|---|
| Plasma cGMP, pmol/ml | 7.4 (3.3) [6.5; 4.2‒11.4] | 7.2 (2.6) [6.8; 4.5‒10.6] |
| Plasma ADMA, µg/l | 109 (25) [110; 77‒143] | 112 (23) [109; 88‒139] |
| Plasma SDMA, µg/l | 108 (30) [101; 78‒133] | 102 (20) [99; 82‒130] |
| Plasma p-ERK, % | 57 (6) [58; 49‒64] | 57 (8) [58; 47‒65] |
| Plasma p-VASP, % | 59 (11) [60; 45‒73] | 59 (13) [61; 44‒72] |
| Serum sPECAM-1, ng/ml | 77.3 (17.1) [75.6; 56.6‒101.1] | 81.6 (22.2) [81.6; 55.0‒107.0] |
| Serum CXCL-4, mg/l | 8.8 (2.9) [9.0; 5.8‒12.0] | 9.2 (2.5) [9.4; 6.7‒12.3] |
| Plasma hsCRP, mg/l | 4.8 (6.7) [2.8; 0.5‒11.4]c,d | 4.1 (6.7) [1.7; 0.3‒10.1]c,e |
| Serum sE-selectin, µg/l | 45.9 (18.7) [43.7; 22.5‒73.1] | 46.1 (18.2) [44.2; 24.8‒71.2] |
| αSMA, VASa | 19.3 (24.0) [5.0; 0.0‒51.0] | 17.0 (27.4) [2.0; 0.0‒68.0] |
| Skin thickness, µmb | 1708 (647) [1696; 966–2403] | 1750 (504) [1749; 1275–2451] |
Measured with endpoints of 0 mm (no αSMA stain) and 100 mm (bright/diffuse αSMA stain) within each skin biopsy sample.
Skin thickness was defined as the distance from the granular layer to the junction between the dermis and subcutaneous fat, assessed in biopsy specimens.
Four values in the placebo arm and nine values in the riociguat arm were below the LLOQ, and were imputed at the LLOQ (0.3 mg/l).
Maximum: 35.6 mg/l.
Maximum: 40.8 mg/l. ADMA: asymmetric dimethylarginine; αSMA: α-smooth muscle actin; cGMP: cyclic guanosine monophosphate; CXCL-4: CXC motif chemokine ligand 4; hsCRP: high-sensitivity CRP; LLOQ, lower limit of quantification; p-ERK: phospho-extracellular signal-regulated kinase; p-VASP: phospho-vasodilator-stimulated phosphoprotein; SDMA: symmetric dimethylarginine; sE-selectin: soluble E-selectin; sPECAM-1: soluble platelet endothelial cell adhesion molecule-1; VAS: visual analogue scale.
Immunohistochemical αSMA status was unavailable for two patients in the placebo group and one patient in the riociguat group. Overall, 32% of patients with data available (riociguat, 34%; placebo, 31%) had no αSMA-positive cells, and of patients who were αSMA-negative, ≥95% were anti-RNA polymerase III-negative (assessed using immunofluorescence), while 23–37% of those who were αSMA-positive were anti-RNA polymerase III-positive (Supplementary Table S1, available at Rheumatology online). Patients who were αSMA-negative had lower baseline hsCRP levels than patients who were αSMA-positive (Supplementary Table S1, available at Rheumatology online).
Changes in biomarkers of NO–sGC–cGMP pathway engagement
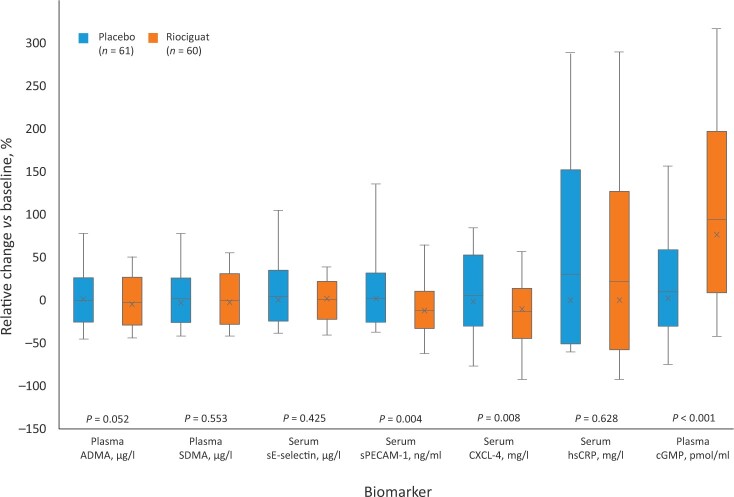
Percentage changes in biomarker levels from baseline to week 14 are shown in Fig. 1. Mean (s.d.) plasma cGMP (assessed by radio-immunoassay) increased from 7.22 (2.57) pmol/ml to 12.92 (5.24) pmol/ml with riociguat, and from 7.44 (3.34) pmol/ml to 7.50 (3.02) pmol/ml with placebo (mean [s.d.] increase of 94 [78]% and 10 [39]%, respectively; P < 0.001 riociguat vs placebo). There were no significant differences between treatment groups in changes in ADMA or SDMA (assessed by HPLC–MS), or in immunohistochemistry-assessed p-ERK or p-VASP, from baseline to week 14 (data not shown). Absolute changes in biomarkers are shown in Supplementary Table S2, available at Rheumatology online.
Figure 1.
Relative changes in serum and plasma biomarkers in riociguat and placebo groups from baseline to week 14. P-values were generated by exploratory t-test and are for information only. Vertical lines: maximum and minimum values; top of box: 90 percentile; ×: median change; horizontal lines: mean change; bottom of box:10 percentile. ADMA: asymmetric dimethylarginine; cGMP: cyclic guanosine monophosphate; CXCL-4: CXC motif chemokine ligand 4; hsCRP: high-sensitivity CRP; SDMA: symmetric dimethylarginine; sE-selectin: soluble E-selectin; sPECAM-1: soluble platelet endothelial cell adhesion molecule-1
Changes in cGMP levels from baseline to week 14 correlated with changes in the riociguat area under the plasma concentration−time curve (r = 0.489; P = 0.001), maximum plasma concentration (r = 0.485; P = 0.008) and trough plasma concentration (r = 0.496; P = 0.001) (Fig. 2). Pharmacokinetic parameters for riociguat are shown in Supplementary Table S3, available at Rheumatology online.
Figure 2.
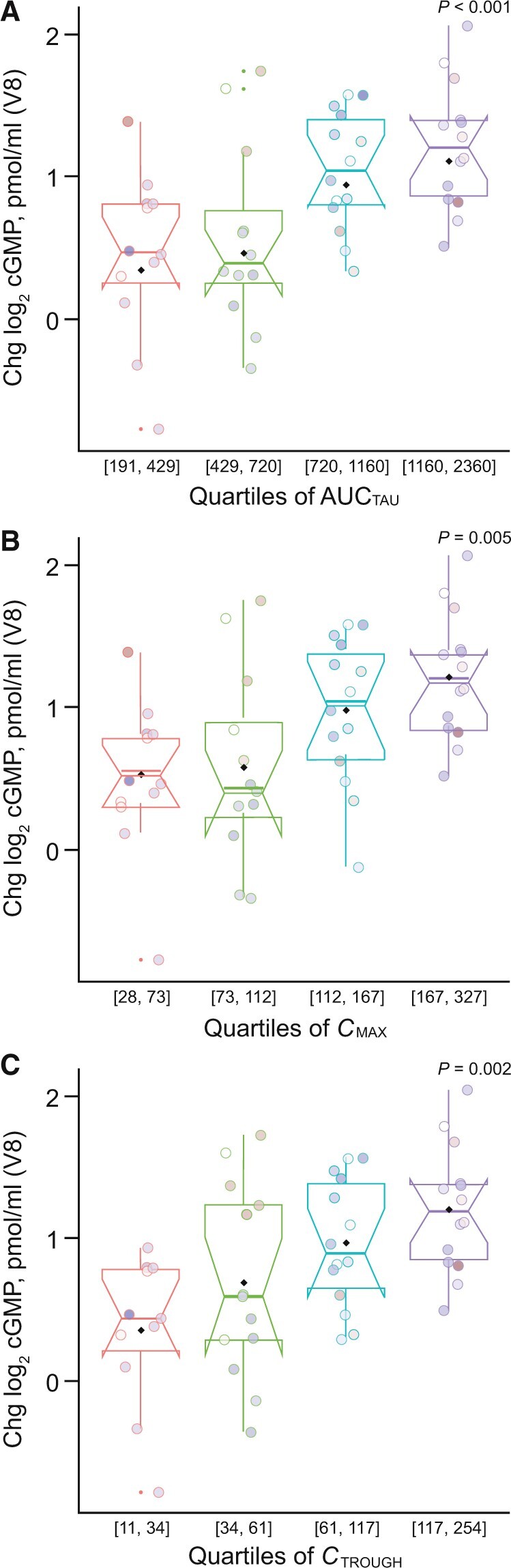
Changes in plasma cGMP to week 14 in riociguat arm by week 14 riociguat concentration quartiles. (A) AUCTAU, (B) CMAX, and (C) CTROUGH. AUCTAU: area under the plasma concentration−time curve. cGMP: cyclic guanosine monophosphate; Chg: change; CMAX: maximum plasma concentration; CTROUGH: trough plasma concentration; V8: study visit 8 (week 14). Circles show values for individual patients. Values in parentheses on x-axes are the upper and lower limits of each quartile. Shading of data points is for visualization only. P-values were generated by exploratory t-test and are for information only.
Changes in biomarkers of disease activity
At week 14, sPECAM-1 and CXCL-4, assessed using ELISA, were reduced in the riociguat group compared with placebo (Fig. 1): mean (s.d.) change in sPECAM-1 was –11.91 (20.42)% in the riociguat group and 2.18 (27.59)% in the placebo group (P = 0.004) and mean (s.d.) change in CXCL-4 was –13.56 (27.36)% in the riociguat group and 5.74 (35.42)% in the placebo group (P = 0.008). Changes in immunoturbidimetry-assessed hsCRP or ELISA-assessed sE-selectin did not differ significantly between the riociguat and placebo groups (see Supplementary Data S1, available at Rheumatology online [p. 3 and Supplementary Table S2]).
mRNA markers of fibrosis (collagen 1A1, 1A2 and 3A1, cartilage oligomeric matrix protein, thrombospondin-1, and fibronectin), assessed in skin biopsies using reverse transcription–quantitative real-time PCR, were highly correlated with each other; however, the changes in these biomarkers did not differ significantly between treatment groups (data not shown). No significant changes in collagen 1A1, 1A2 or 3A1 were seen between baseline and week 14 with riociguat (mean fold-changes 1.27, 1.24 and 1.21, respectively) or placebo (mean fold-changes 0.96, 1.11 and 1.05, respectively).
Prognostic significance of biomarkers (data from placebo arm)
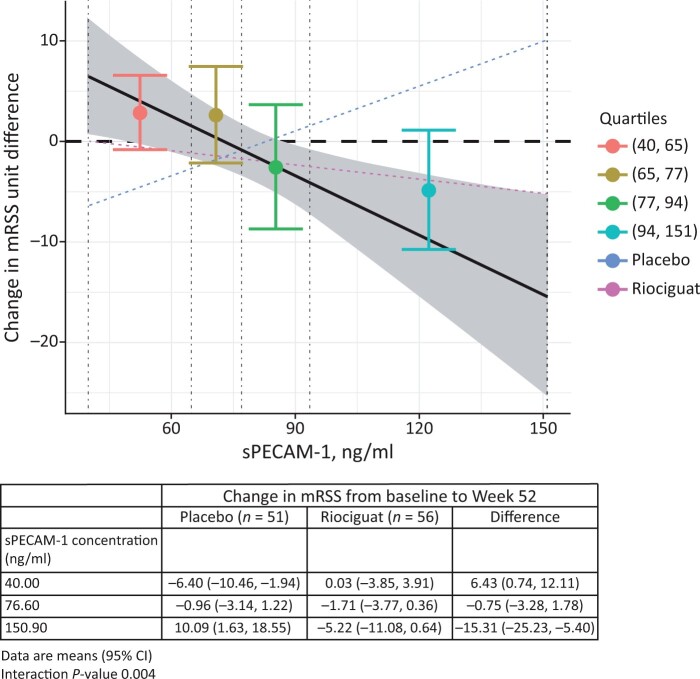
In the placebo arm, there were no correlations with r > 0.3 between biomarker values including cGMP at baseline or week 14 and the change in mRSS from baseline to week 52 (apart from change in cGMP; r = 0.315) (Supplementary Table S4, available at Rheumatology online). In the placebo arm, higher baseline sPECAM-1 was associated with an increase in mRSS from baseline to week 52 (interaction P = 0.004; correlation between baseline sPECAM-1 and change in mRSS: 0.275) (Fig 3). Other biomarkers showed no prognostic potential with respect to mRSS at week 52 in the placebo arm. Patients in the placebo arm who were αSMA-positive (n = 35) had numerically less improvement in mRSS at week 52 than those who were αSMA-negative (n = 16), with a prognostic effect of −3.82 (95% CI: −8.81, 1.18); P = 0.131.
Figure 3.
Relationship between baseline sPECAM-1 quartiles and treatment difference between riociguat and placebo (change in mRSS at week 52). Vertical dashed lines indicate quartiles of sPECAM concentration; grey shading indicates 95% CI of the linear regression line. Interaction P-value obtained from an analysis of covariance model adjusting for baseline mRSS and region, treatment, continuous sPECAM, and interaction between treatment and sPECAM. mRSS: modified Rodnan skin score; sPECAM: soluble platelet endothelial cell adhesion molecule
Association of biomarker levels at baseline with effects of riociguat on mRSS at week 52
Higher baseline levels of sPECAM-1 were associated with a greater reduction of mRSS at week 52 with riociguat vs placebo (interaction P = 0.004) (Fig. 3). Baseline levels of hsCRP, CXCL-4, p-VASP or fold-change of fibronectin had no clear predictive value, showing interaction P > 0.05 (P = 0.06 for hsCRP). With the exception of CXCL-4 (correlation 0.377) there were no correlations >0.3 between baseline biomarkers (including cGMP) and the change in mRSS from baseline to week 52 in the riociguat group (Supplementary Table S5, available at Rheumatology online).
Associations of αSMA-positive cells at baseline with effects of riociguat on mRSS at week 52
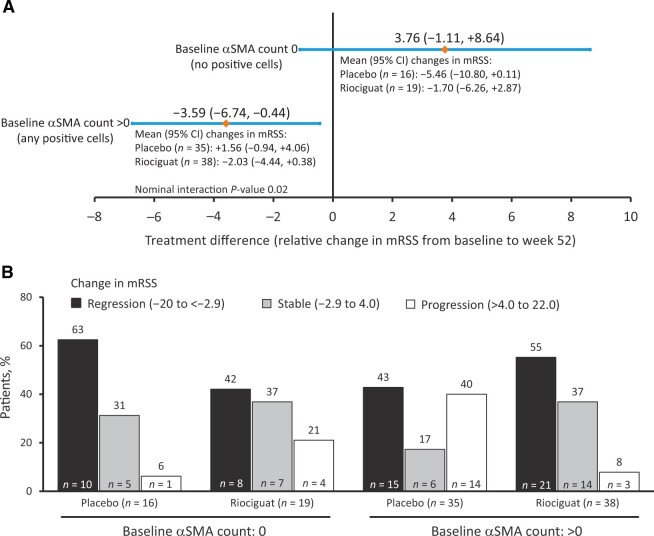
In patients showing no αSMA-positive cells, the treatment difference between riociguat and placebo for mRSS at week 52 was 3.76 (95% CI: −1.11, 8.64); in patients with αSMA-positive cells the difference was −3.59 (95% CI: −6.74, 0.44) (interaction P = 0.02) (Fig. 4A). Progression of mRSS (increase of 4–22 units) to week 52 was observed in 40% of patients with αSMA-positive cells at baseline in the placebo arm and in 8% of such patients in the riociguat arm (Fig. 4B).
Figure 4.
Treatment difference between placebo and riociguat (A) and changes in mRSS (B) by baseline αSMA status. Interaction P-value obtained from an analysis of covariance model adjusting for baseline mRSS and region, treatment, continuous αSMA, and interaction between treatment and αSMA. αSMA: α-smooth muscle actin; mRSS: modified Rodnan skin score
Among patients with αSMA-positive cells, the treatment difference of change in mRSS for riociguat compared with placebo was −4.90 (95% CI: −10.04, 0.23) in those with baseline mRSS 17–22 units, and 0.75 (95% CI: −2.89, 4.4) if baseline mRSS was 10 to <17 units. These observations suggest a reduction in mRSS with riociguat in patients with αSMA-positive cells and higher mRSS at baseline. The effect of αSMA-positive cells at baseline on the response to riociguat was seen in patients who were also positive for anti-RNA polymerase III or anti-Scl-70 with a treatment difference of −5.6 (95% CI: −9.28, 1.91; interaction P = 0.005 for baseline αSMA cell status), but not seen in those who were also both anti-RNA polymerase III and anti-Scl-70-negative (treatment difference: 0.42; 95% CI: −4.96, 5.82). Analysis of the change in mRSS at week 52 in relation to changes in αSMA-positive cell counts at week 14 categorized into quartiles showed no clear association (interaction P = 0.186; Supplementary Table S6, available at Rheumatology online).
Association of changes in biomarkers at week 14 with effects of riociguat on mRSS at week 52
There was no clear evidence of an association between the change in mRSS at week 52 and changes in αSMA-positive cell counts at week 14 categorized into quartiles (see Supplementary Table S6, available at Rheumatology online). Changes from baseline to week 14 in other biomarkers including cGMP had no clear relationship with change in mRSS (all correlations <0.3; Supplementary Table S5, available at Rheumatology online). Fold-changes of mRNA for collagen 1A1, cartilage oligomeric matrix protein and fibronectin showed correlations of 0.339, 0.306 and 0.358, respectively, with change from baseline to week 52 in mRSS in the riociguat group. Correlations for other mRNA fold-changes were <0.3.
Associations of baseline levels, or changes in biomarker levels at week 14, with effects of riociguat on other endpoints
Baseline levels of biomarkers or their changes from baseline to week 14 showed no clear relationship with changes from baseline to week 52 in forced vital capacity (FVC) % predicted, carbon monoxide diffusing capacity (DLCO) % predicted, HAQ-DI, digital ulcer burden or (except for two assessments of sPECAM-1) Raynaud’s disease assessments (see Supplementary Data S1, available at Rheumatology online, pp. 9–10). The baseline level of cGMP or its change from baseline to week 14 also had no clear relationship with these endpoints (correlations <0.3).
Discussion
This analysis examined several biomarkers in patients with early treatment-naïve dcSSc treated with riociguat or placebo in RISE-SSc [12]. Elevation of plasma cGMP with riociguat indicated engagement with the NO–sGC–cGMP pathway and correlated with pharmacokinetic variables of riociguat. By contrast, changes in ADMA, SDMA, p-ERK and p-VASP were similar between riociguat and placebo. This may be due to these molecules being further downstream than cGMP, and thus less direct measures of engagement [35].
In RISE-SSc, despite recruitment of a very early progressive SSc population, many patients had αSMA-negative skin biopsies (vascular and glandular tissue were excluded from the counts). Patients with negative αSMA counts have been observed previously, reflecting the heterogeneity of myofibroblast activation in SSc [33, 34]. In addition, extracellular matrix deposition in early SSc may be due to other cell–cell interactions such as endothelial-to-mesenchymal transition [36].
In our analysis, higher baseline serum sPECAM-1 levels were associated with an increase in mRSS from baseline to week 52 (Fig. 3). PECAM-1 is involved in the transmigration of leucocytes into tissues [26, 37]. Compared with controls, serum levels of sPECAM-1 are significantly elevated in patients with dcSSc or limited SSc (lSSc), and significantly more so in the latter [26]. Elevated serum sPECAM-1 was associated with lSSc of relatively early onset and with lower frequency and severity of pulmonary fibrosis, suggesting that sPECAM-1 elevation may protect against development of skin sclerosis and pulmonary fibrosis in SSc [26]. Studies in PECAM-1-deficient animal models suggest that PECAM-1 has a protective action [27], and transition of endothelial cells from patients with SSc toward a mesenchymal phenotype is associated with reduced PECAM-1 expression [38]. It is unclear why elevated serum sPECAM-1 was associated with progression of skin fibrosis in our study but it may reflect a compensatory response to disease activity. Also, serum measurements might not reflect intracellular levels or expression in specific tissues.
αSMA positivity at baseline was associated with a greater effect of riociguat on mRSS at week 52, the primary end point of RISE-SSc. Anti-Scl-70 or anti-RNA polymerase III antibodies are associated with poor outcomes in SSc [14, 39]. In subgroup analyses, the greater change in mRSS at week 52 with riociguat in patients with vs without αSMA-positive cells at baseline was only apparent in those who were also anti-RNA polymerase III- or anti-Scl-70-positive. Thus, the presence of anti-RNA polymerase III antibodies may have been the driver for the effect of riociguat in αSMA-positive patients. Small patient numbers (only one patient was anti-RNA polymerase III-positive and αSMA-negative) preclude further analysis. The current results should be viewed in terms of signal detection, and any subgroup results should be confirmed by further analyses. We were unsurprised to see a lack of association between change in αSMA-positive cells at week 14 and change in DLCO % predicted or FVC % predicted given the known dissociation of skin and lung progression in dcSSc [40, 41]. However, our results indicate that the presence of αSMA in early SSc has value as a biomarker for progression of skin fibrosis.
CXCL-4 is elevated in SSc, correlating with the presence and progression of complications such as lung fibrosis and PAH, and was therefore evaluated as a marker of dcSSc progression [42]. CXCL-4 mediates fibrosis by transforming endothelial and stromal cells into myofibroblasts with excessive collagen production, whereas absence or blockade of CXCL-4 diminishes tissue fibrosis in numerous models [43]. Levels of CRP correlate with the severity of lung, skin and joint involvement in SSc, and increased levels are associated with shorter survival [44, 45]. In RISE-SSc, at week 14, treatment with riociguat was associated with a decrease in sPECAM-1 and CXCL-4, but not other biomarkers, including hsCRP, which increased. The explanation for the differing responses between biomarkers is unclear, but week 14 may have been too early to observe effects for the other biomarkers. The mechanism of the decrease in CXCL-4 with riociguat is unclear; inhibition of platelet activation appears unlikely since effects of riociguat on platelets have been seen only at concentrations far exceeding those seen in therapy [46]. CXCL-4 is present in platelet granules and is released upon platelet activation. In the current study, the CXCL-4 assay was performed on platelet-poor plasma (see Supplementary Data, available at Rheumatology online [p. 3] for details) to avoid overestimation of ‘physiological’ CXCL-4 levels due to CXCL-4 release from platelets. However, removal of platelets may have been incomplete, and therefore the CXCL-4 results reported here should be viewed with caution.
TGF-β is an important mediator of the fibrotic process in SSc; it promotes endothelial cell activation, differentiation toward mesenchymal cells, and the expression of mesenchymal markers such as αSMA [21, 36, 38]. Riociguat inhibits TGF-β1 signalling [5–7] and could potentially provide benefits in dcSSc by inhibiting endothelial-to-mesenchymal transition. This effect might be expected to be most marked in patients with active endothelial-to-mesenchymal cell transition, and in our study patients with αSMA-positive cells obtained the greatest benefit from riociguat. In the present study, riociguat had no effect on skin collagen markers as measured by fold-change or on αSMA staining, so may not be able to reverse more advanced fibrosis despite its potential effect on TGF-β signalling. There are many mediators of skin fibrosis in SSc and most of these were not significantly changed by riociguat compared with placebo. Injured endothelial cells in SSc produce low levels of NO and endothelial NO synthase [47]; stimulation of the NO–sGC–cGMP pathway by riociguat could therefore improve vascular function. Elevation of CXCL-4 also appears to play a role in peripheral vasculopathy in SSc [48]; reduction of this biomarker by riociguat could be another potential mechanism of benefit.
Several limitations of this study should be considered. Biomarkers were sampled at baseline and week 14; additional sampling would have been valuable, as would analysis of mRSS at other time points. Assessment of αSMA by two reviewers using a 0–100 visual analogue scale has been reported in blinded studies [33, 34], but this technique may have contributed to some of the variation observed. Imputing values for data below or above the limit of quantification may introduce systematic bias. Our analyses did not control for baseline disease severity. Another limitation is the lack of a larger effect of riociguat on mRSS in the main study, although the current results suggest that riociguat may influence mRSS progression in patients with rapidly progressive disease. While RISE-SSc was successful in part in selecting patients at greater risk of skin fibrosis progression [12], our results may reflect low disease activity in some patients. When considering the subgroups according to anti-Scl-70 and anti-RNA polymerase III status, it is important to bear in mind that there is a lack of standardization in clinical care, particularly with regard to Scl-70, but this consideration has limited reference to the current study, in which autoantibodies were assessed in a standardized laboratory. The results might not be generalizable to patients outside the study population (e.g. advanced dcSSc or lSSc).
Overall, αSMA positivity status was most consistently associated with clinical endpoints. The close association with anti-RNA polymerase III-positive status might drive the effects of riociguat in patients with an αSMA-positive cell count at baseline. A nominally significant decrease of CXCL-4 and sPECAM-1 from baseline to week 14 suggests anti-inflammatory properties of riociguat in patients with dcSSc and our findings suggest patients with increased circulating sPECAM-1 at baseline may have a greater response to riociguat. Further research is warranted to clarify the relative importance of biomarkers in dcSSc to aid clinical decision-making. The long-term open-label extension phase of the RISE-SSc trial has recently been reported [49].
Supplementary Material
Acknowledgements
We thank the investigators and patients in the RISE-SSc trial, as well as the Data Monitoring Committee and the Central Adjudication Committee. Medical writing services, provided by Richard Murphy PhD of Adelphi Communications Ltd, Macclesfield, UK, were funded by Bayer AG, Berlin, Germany, in accordance with Good Publication Practice 2022 guidelines.
Contributor Information
Dinesh Khanna, Division of Rheumatology, University of Michigan, Ann Arbor, MI, USA.
Frank Kramer, Research and Development, Pharmaceuticals, Bayer AG, Wuppertal, Germany.
Josef Höfler, Staburo GmbH, Munich, Germany.
Mercedeh Ghadessi, Research and Development, Pharmaceuticals, Bayer AG, Wuppertal, Germany.
Peter Sandner, Research and Development, Pharmaceuticals, Bayer AG, Wuppertal, Germany.
Yannick Allanore, Rheumatology A Department, Cochin Hospital, APAP, Paris Descartes University, Paris, France.
Christopher P Denton, Division of Medicine, Centre for Rheumatology, University College London, London, UK.
Masataka Kuwana, Department of Allergy and Rheumatology, Nippon Medical School Graduate School of Medicine, Tokyo, Japan.
Marco Matucci-Cerinic, Division of Rheumatology, Department of Experimental and Clinical Medicine, University of Firenze, Florence, Italy; Unit of Immunology, Rheumatology, Allergy and Rare Diseases, IRCCS San Raffaele Hospital, Milan, Italy.
Janet E Pope, Division of Rheumatology, Schulich School of Medicine, University of Western Ontario, London, ON, Canada.
Tatsuya Atsumi, Department of Rheumatology, Endocrinology and Nephrology, Faculty of Medicine and Graduate School of Medicine, Hokkaido University, Sapporo, Japan.
Radim Bečvář, Institute of Rheumatology, Department of Rheumatology, 1st Faculty of Medicine, Charles University, Prague, Czech Republic.
László Czirják, Department of Rheumatology and Immunology, Medical School, University of Pécs, Pécs, Hungary.
Ellen De Langhe, Laboratory of Tissue Homeostasis and Disease, Skeletal Biology and Engineering Research Center, Division of Rheumatology, Department of Development and Regeneration, KU Leuven, University Hospitals Leuven, Leuven, Belgium.
Eric Hachulla, Department of Internal Medicine and Clinical Immunology, Referral Centre for Centre for Rare Systemic Autoimmune Diseases North and North-West of France, CHU Lille, University of Lille, Inserm, U1286 - INFINITE—Institute for Translational Research in Inflammation, Lille, France.
Tomonori Ishii, Clinical Research, Innovation and Education Center, Tohoku University, Sendai, Japan.
Osamu Ishikawa, Department of Dermatology, Gunma University Postgraduate School of Medicine, Maebashi, Japan.
Sindhu R Johnson, Division of Rheumatology, Department of Medicine, Toronto Western Hospital, University Health Network, Mount Sinai Hospital, University of Toronto, Toronto Scleroderma Research Program, Toronto, ON, Canada.
Valeria Riccieri, Department of Clinical, Internal, Anesthesiological and Cardiovascular Sciences, Sapienza University of Rome, Rome, Italy.
Elena Schiopu, Division of Rheumatology, Medical College of Georgia at Augusta University, Augusta, GA, USA.
Richard M Silver, Division of Rheumatology and Immunology, Medical University of South Carolina, Charleston, SC, USA.
Vanessa Smith, Department of Internal Medicine, Ghent University, Belgium and Department of Rheumatology, Ghent University Hospital, Belgium, and Unit for Molecular Immunology and Inflammation, VIB Inflammation Research Center, Ghent, Belgium.
Chiara Stagnaro, Rheumatology Unit, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy.
Virginia Steen, Division of Rheumatology, Department of Medicine, Georgetown University Medical Center, Washington, DC, USA.
Wendy Stevens, Department of Rheumatology, St Vincent's Hospital Melbourne, Melbourne, VIC, Australia.
Gabriella Szücs, Department of Rheumatology, University of Debrecen, Debrecen, Hungary.
Marie-Elise Truchetet, Department of Rheumatology, CHU Bordeaux, Bordeaux, France.
Melanie Wosnitza, Research and Development, Pharmaceuticals, Bayer AG, Wuppertal, Germany.
Oliver Distler, Department of Rheumatology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the European Federation of Pharmaceutical Industries and Associations and Pharmaceutical Research and Manufacturers of America principles for responsible clinical trial data sharing, pertaining to scope, time point and process of data access. Bayer commits to sharing upon request from qualified scientific and medical researchers, patient-level clinical trial data, study-level clinical trial data and protocols from clinical trials in patients for medicines and indications approved in the US and European Union as necessary for performing legitimate research. This commitment applies to data on new medicines and indications that have been approved by the European Union and US regulatory agencies on or after 1 January 2014. Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to perform further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the study sponsors section of the portal. Data access will be granted to anonymized patient-level data, protocols and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
Funding
This work was supported jointly by Bayer AG and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Disclosure statement: O.D. has/had consultancy relationships with and/or has received research funding from, or has served as a speaker in the area of potential treatments for systemic sclerosis and its complications in the last 3 years for AbbVie, Acceleron, Amgen, AnaMar, Arxx Therapeutics, Baecon, Bayer AG, Blade, Boehringer Ingelheim, ChemomAb, Corbus, CSL Behring, Galapagos NV, GlaxoSmithKline, Glenmark, Horizon (Curzion), Inventiva, iQvia, Italfarmaco, Kymera, Medac, Medscape, Merck Sharp & Dohme, Mitsubishi Tanabe, Novartis, Pfizer, Roche, Roivant, Sanofi, Serodapharm, Target Bioscience AG, Topadur and UCB. O.D. is named on a patent issued for ‘mir-29 for the treatment of systemic sclerosis’ (US8247389, EP2331143). F.K. is an employee of Bayer AG and holds shares in Bayer AG. J.H. is an employee of Staburo GmbH, Germany, contracted by Bayer AG. M.G. is an employee of Bayer AG. P.S. is an employee of Bayer AG. Y.A. has received grant/research support from Bristol Myers Squibb, Inventiva, Roche-Genentech and Sanofi S.A.; consultancy for Actelion, Bayer AG, Bristol Myers Squibb and Boehringer Ingelheim. C.P.D. has received grant/research support from GlaxoSmithKline, CSL Behring and Inventiva; consultancy for Medscape, Roche-Genentech, Actelion, GlaxoSmithKline, Sanofi S.A., Inventiva, CSL Behring, Boehringer Ingelheim, Corbus Pharmaceuticals, Acceleron Pharma, Inc., Horizon Therapeutics (Curizon) and Bayer AG. M.K. has received grant/research support from Boehringer Ingelheim, Ono Pharmaceutical Co., Ltd and MBL; consultancy for Boehringer Ingelheim, Bayer AG, Corbus Pharmaceuticals, Galapagos NV and Mochida Pharmaceutical Co., Ltd; speaker fees from AbbVie Inc., Asahi Kasei Pharma, Chugai Pharmaceutical Co., Ltd, Eisai Co., Ltd, GlaxoSmithKline, Janssen, Nippon Shinyaku Co., Ltd, Mitsubishi Tanabe Pharma Corporation, Boehringer Ingelheim and Ono Pharmaceutical Co., Ltd. M.M-C. has received grant/research support from Actelion, MSD and Bristol Myers Squibb; speaker fees from Actelion, Eli Lilly & Company and Boehringer Ingelheim. J.E.P. has received grant/research support from AbbVie Inc., Bristol Myers Squibb, Eli Lilly & Company, Merck & Co., Inc., Roche, Seattle Genetics (now Seagen Inc.) and UCB; consultancy for AbbVie Inc., Actelion, Amgen Inc., Bayer AG, Boehringer Ingelheim, Bristol Myers Squibb, Eicos Sciences Inc., Eli Lilly & Company, Emerald Health Pharmaceuticals, Gilead Sciences, Inc., Janssen, Merck & Co., Inc., Novartis, Pfizer Inc., Roche, Sandoz, Sanofi S.A. and UCB; speakers fees from UCB. T.A. has received grant/research support from Eli Lilly Japan K.K., Alexion Pharmaceuticals, Inc., Bristol Myers Squibb Co., AbbVie Inc., Daiichi Sankyo Co., Ltd, Pfizer Inc., Chugai Pharmaceutical Co., Ltd, Mitsubishi Tanabe Pharma Corporation and Astellas Pharma Inc.; consultancy for Gilead Sciences, Inc., Eli Lilly Japan K.K., UCB Japan Co. Ltd, AbbVie Inc., Daiichi Sankyo Co., Ltd, Pfizer Inc. and Chugai Pharmaceutical Co., Ltd; speakers fees from Eli Lilly Japan K.K., UCB Japan Co. Ltd, Bristol Myers Squibb Co., AbbVie Inc., Eisai Co. Ltd, Otsuka Pharmaceutical Co., Ltd, Daiichi Sankyo Co., Ltd, Pfizer Inc., Chugai Pharmaceutical Co., Ltd, Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd and Astellas Pharma Inc. R.B. has received consultancy fees for Actelion and Roche. L.C. has received consultancy fess from Actelion, Boehringer Ingelheim, Roche-Genentech, Eli Lilly & Company, Medac Pharma, Novartis, Pfizer Inc. and Bayer AG. E.D.L. has/had a consultancy relationship and/or speaker fees from Boehringer Ingelheim Pharma GmbH & Co., GlaxoSmithKline, AstraZeneca, Argenx, Amgen Inc. and Novartis. V.Sm. has received grant/research support from the Research Foundation Flanders, the Belgian Fund for Scientific Research, Boehringer Ingelheim Pharma GmbH & Co. and Janssen-Cilag NV; consultancy for Boehringer Ingelheim Pharma GmbH & Co. and Janssen-Cilag NV; speakers fees from Actelion Pharmaceuticals Ltd, Boehringer Ingelheim Pharma GmbH & Co., Janssen-Cilag NV and UCB Biopharma Sprl. V.St has received grant/research support from Boehringer Ingelheim, Corbus Pharmaceuticals, CSL Behring, Eicos Sciences Inc., Galapagos NV, Immune Tolerance Network and Reata Pharmaceuticals Inc.; consultancy for Boehringer Ingelheim, Corbus Pharmaceuticals, CSL Behring, Eicos Sciences Inc. and Forbius. M.W. is an employee of Bayer AG. D.K. is a shareholder of Eicos Sciences, Inc./CiVi Biopharma, Inc.; and a consultant for Acceleron Pharma, Inc., AstraZeneca, Actelion, Bayer AG, Boehringer Ingelheim, Bristol Myers Squibb, Horizon Therapeutics, Mitsubishi Tanabe Pharma Corporation, Prometheus Biosciences and Roche/Genentech. M-E.T. has received consultancy and speaker fees from Boehringer Ingelheim and Eli Lilly & Company. G.S. reports personal fees from Boehringer Ingelheim, Janssen-Cilag, Roche-Genentech, Eli Lilly & Company, Novartis, CSL Behring, Pfizer Inc., Berlin-Chemie AG/Menarini and Sager Pharma, outside the submitted work. W.S. has received speaker fees, advisory board fees and research grants from Jansen and GlaxoSmithKline and advisory board fees from Boehringer Ingelheim. E.S. has received grant/research support from Bayer AG for the RISE-SSc study. S.R.J. was a site investigator for trials sponsored by Boehringer Ingelheim and Corbus; and served on advisory boards sponsored by Boehringer Ingelheim and Ikaria, Inc. E.H. has received grants and personal fees from Actelion and GlaxoSmithKline, and personal fees from Boehringer Ingelheim, Roche and Sanofi-Genzyme. T.I. has received personal fees from Janssen, Asahi Kasei Pharma, Ono Pharmaceutical, Ayumi Pharmaceutical, Pfizer, Astellas, Daiichi Sankyo, Chugai Pharmaceutical, Sanofi, AbbVie, Bristol Myers Squibb, Mitsubishi Tanabe Pharma and Eisai. R.M.S. has received research funding and consultancy fees from Boehringer Ingelheim, and consultancy fees from Corbus, Forbius and Medtelligence. O.I., V.R. and C.S. declare no potential conflicts of interest relevant to this article.
References
- 1. Denton CP, Khanna D. Systemic sclerosis. Lancet 2017;390:1685–99. [DOI] [PubMed] [Google Scholar]
- 2. Khanna D, Furst DE, Clements PJ et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J Scleroderma Relat Disord 2017;2:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Varga J, Trojanowska M, Kuwana M. Pathogenesis of systemic sclerosis: recent insights of molecular and cellular mechanisms and therapeutic opportunities. J Scleroderma Relat Disord 2017;2:137–52. [Google Scholar]
- 4. Khanna D, Lescoat A, Roofeh D et al. Systemic sclerosis-associated interstitial lung disease: how to incorporate two Food and Drug Administration-approved therapies in clinical practice. Arthritis Rheumatol 2021;74:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dees C, Beyer C, Distler A et al. Stimulators of soluble guanylate cyclase (sGC) inhibit experimental skin fibrosis of different aetiologies. Ann Rheum Dis 2015;74:1621–5. [DOI] [PubMed] [Google Scholar]
- 6. Sandner P, Zimmer DP, Milne GT et al. Soluble guanylate cyclase stimulators and activators. Handb Exp Pharmacol 2021;264:425–94. [DOI] [PubMed] [Google Scholar]
- 7. Sandner P, Stasch JP. Anti-fibrotic effects of soluble guanylate cyclase stimulators and activators: a review of the preclinical evidence. Respir Med 2017;122(Suppl 1):S1–S9. [DOI] [PubMed] [Google Scholar]
- 8. Ghofrani HA, Galiè N, Grimminger F et al. ; PATENT-1 Study Group. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013;369:330–40. [DOI] [PubMed] [Google Scholar]
- 9. Ghofrani HA, Grimminger F, Grünig E et al. Predictors of long-term outcomes in patients treated with riociguat for pulmonary arterial hypertension: data from the PATENT-2 open-label, randomised, long-term extension trial. Lancet Respir Med 2016;4:361–71. [DOI] [PubMed] [Google Scholar]
- 10. Humbert M, Coghlan JG, Ghofrani HA et al. Riociguat for the treatment of pulmonary arterial hypertension associated with connective tissue disease: results from PATENT-1 and PATENT-2. Ann Rheum Dis 2017;76:422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huntgeburth M, Kießling J, Weimann G et al. Riociguat for the treatment of raynaud's phenomenon: a single-dose, double-blind, randomized, placebo-controlled cross-over pilot study (DIGIT). Clin Drug Investig 2018;38:1061–9. [DOI] [PubMed] [Google Scholar]
- 12. Khanna D, Allanore Y, Denton CP et al. Riociguat in patients with early diffuse cutaneous systemic sclerosis (RISE-SSc): randomised, double-blind, placebo-controlled multicentre trial. Ann Rheum Dis 2020;79:618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. FDA-NIH Biomarker Working Group, Silver Spring (MD), Food and Drug Administration (US), Bethesda (MD), (US) NIoH. BEST (Biomarkers, EndpointS, and other Tools) Resource. 2016. https://www.ncbi.nlm.nih.gov/books/NBK326791/ (13 July 2022, date last accessed). [PubMed]
- 14. Herrick AL, Peytrignet S, Lunt M et al. Patterns and predictors of skin score change in early diffuse systemic sclerosis from the European Scleroderma Observational Study. Ann Rheum Dis 2018;77:563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dobrota R, Maurer B, Graf N et al. ; EUSTAR Coauthors. Prediction of improvement in skin fibrosis in diffuse cutaneous systemic sclerosis: a EUSTAR analysis. Ann Rheum Dis 2016;75:1743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maurer B, Graf N, Michel BA et al. ; EUSTAR Co-authors. Prediction of worsening of skin fibrosis in patients with diffuse cutaneous systemic sclerosis using the EUSTAR database. Ann Rheum Dis 2015;74:1124–31. [DOI] [PubMed] [Google Scholar]
- 17. van den Hoogen F, Khanna D, Fransen J et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747–55. [DOI] [PubMed] [Google Scholar]
- 18. LeRoy EC, Medsger TA Jr. Criteria for the classification of early systemic sclerosis. J Rheumatol 2001;28:1573–6. [PubMed] [Google Scholar]
- 19. Tain YL, Hsu CN. Toxic dimethylarginines: asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA). Toxins (Basel) 2017;9:92.28272322 [Google Scholar]
- 20. Dooley A, Gao B, Bradley N et al. Abnormal nitric oxide metabolism in systemic sclerosis: increased levels of nitrated proteins and asymmetric dimethylarginine. Rheumatology (Oxford) 2006;45:676–84. [DOI] [PubMed] [Google Scholar]
- 21. Samuel GH, Bujor AM, Nakerakanti SS, Hant FN, Trojanowska M. Autocrine transforming growth factor beta signaling regulates extracellular signal-regulated kinase 1/2 phosphorylation via modulation of protein phosphatase 2A expression in scleroderma fibroblasts. Fibrogenesis Tissue Repair 2010;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Y, Leask A, Abraham DJ et al. Heparan sulfate-dependent ERK activation contributes to the overexpression of fibrotic proteins and enhanced contraction by scleroderma fibroblasts. Arthritis Rheum 2008;58:577–85. [DOI] [PubMed] [Google Scholar]
- 23. Becker EM, Schmidt P, Schramm M et al. The vasodilator-stimulated phosphoprotein (VASP): target of YC-1 and nitric oxide effects in human and rat platelets. J Cardiovasc Pharmacol 2000;35:390–7. [DOI] [PubMed] [Google Scholar]
- 24. Utsunomiya A, Oyama N, Hasegawa M. Potential biomarkers in systemic sclerosis: a literature review and update. J Clin Med 2020;9:3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abignano G, Del Galdo F. Biomarkers as an opportunity to stratify for outcome in systemic sclerosis. Eur J Rheumatol 2020;7:S193–S202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sato S, Komura K, Hasegawa M, Fujimoto M, Takehara K. Clinical significance of soluble CD31 in patients with systemic sclerosis (SSc): association with limited cutaneous SSc. J Rheumatol 2001;28:2460–5. [PubMed] [Google Scholar]
- 27. Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol 2007;27:2514–23. [DOI] [PubMed] [Google Scholar]
- 28. van Caam A, Vonk M, van den Hoogen F, van Lent P, van der Kraan P. Unraveling SSc pathophysiology; the myofibroblast. Front Immunol 2018;9:2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Y, Leask A, Abraham DJ et al. Thrombospondin 1 is a key mediator of transforming growth factor beta-mediated cell contractility in systemic sclerosis via a mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK)-dependent mechanism. Fibrogenesis Tissue Repair 2011;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hinz B, Phan SH, Thannickal VJ et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol 2012;180:1340–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rajkumar VS, Howell K, Csiszar K et al. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res Ther 2005;7:R1113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farina G, Lemaire R, Pancari P et al. Cartilage oligomeric matrix protein expression in systemic sclerosis reveals heterogeneity of dermal fibroblast responses to transforming growth factor beta. Ann Rheum Dis 2009;68:435–41. [DOI] [PubMed] [Google Scholar]
- 33. Kissin EY, Merkel PA, Lafyatis R. Myofibroblasts and hyalinized collagen as markers of skin disease in systemic sclerosis. Arthritis Rheum 2006;54:3655–60. [DOI] [PubMed] [Google Scholar]
- 34. Rice LM, Padilla CM, McLaughlin SR et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest 2015;125:2795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dooley A, Bruckdorfer KR, Abraham DJ. Modulation of fibrosis in systemic sclerosis by nitric oxide and antioxidants. Cardiol Res Pract 2012;2012:521958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Di Benedetto P, Ruscitti P, Berardicurti O et al. Endothelial-to-mesenchymal transition in systemic sclerosis. Clin Exp Immunol 2021;205:12–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fawcett J, Buckley C, Holness CL et al. Mapping the homotypic binding sites in CD31 and the role of CD31 adhesion in the formation of interendothelial cell contacts. J Cell Biol 1995;128:1229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cipriani P, Di Benedetto P, Ruscitti P et al. The endothelial-mesenchymal transition in systemic sclerosis is induced by endothelin-1 and transforming growth factor-beta and may be blocked by macitentan, a dual endothelin-1 receptor antagonist. J Rheumatol 2015;42:1808–16. [DOI] [PubMed] [Google Scholar]
- 39. Hamaguchi Y. Autoantibody profiles in systemic sclerosis: predictive value for clinical evaluation and prognosis. J Dermatol 2010;37:42–53. [DOI] [PubMed] [Google Scholar]
- 40. Distler O, Highland KB, Gahlemann M et al. ; SENSCIS Trial Investigators. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med 2019;380:2518–28. [DOI] [PubMed] [Google Scholar]
- 41. Wu W, Jordan S, Graf N et al. ; EUSTAR Collaborators. Progressive skin fibrosis is associated with a decline in lung function and worse survival in patients with diffuse cutaneous systemic sclerosis in the European Scleroderma Trials and Research (EUSTAR) cohort. Ann Rheum Dis 2019;78:648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Bon L, Affandi AJ, Broen J et al. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N Engl J Med 2014;370:433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Affandi AJ, Carvalheiro T, Ottria A et al. CXCL4 drives fibrosis by promoting several key cellular and molecular processes. Cell Rep 2022;38:110189. [DOI] [PubMed] [Google Scholar]
- 44. Liu X, Mayes MD, Pedroza C et al. Does C-reactive protein predict the long-term progression of interstitial lung disease and survival in patients with early systemic sclerosis? Arthritis Care Res (Hoboken) 2013;65:1375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hoffmann-Vold A, Allanore Y, Alves M et al. ; EUSTAR Collaborators. Progressive interstitial lung disease in patients with systemic sclerosis-associated interstitial lung disease in the EUSTAR database. Ann Rheum Dis 2021;80:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reiss C, Mindukshev I, Bischoff V et al. The sGC stimulator riociguat inhibits platelet function in washed platelets but not in whole blood. Br J Pharmacol 2015;172:5199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Asano Y, Sato S. Vasculopathy in scleroderma. Semin Immunopathol 2015;37:489–500. [DOI] [PubMed] [Google Scholar]
- 48. Jiang Z, Chen C, Yang S et al. Contribution to the peripheral vasculopathy and endothelial cell dysfunction by CXCL4 in systemic sclerosis. J Dermatol Sci 2021;104:63–73. [DOI] [PubMed] [Google Scholar]
- 49. Distler O, Allanore Y, Denton CP et al. Riociguat in patients with early diffuse cutaneous systemic sclerosis (RISE-SSc): open-label, long-term extension of a phase 2b, randomised, placebo-controlled trial. Lancet Rheum 2023;5:e660–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the European Federation of Pharmaceutical Industries and Associations and Pharmaceutical Research and Manufacturers of America principles for responsible clinical trial data sharing, pertaining to scope, time point and process of data access. Bayer commits to sharing upon request from qualified scientific and medical researchers, patient-level clinical trial data, study-level clinical trial data and protocols from clinical trials in patients for medicines and indications approved in the US and European Union as necessary for performing legitimate research. This commitment applies to data on new medicines and indications that have been approved by the European Union and US regulatory agencies on or after 1 January 2014. Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to perform further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the study sponsors section of the portal. Data access will be granted to anonymized patient-level data, protocols and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.