Abstract
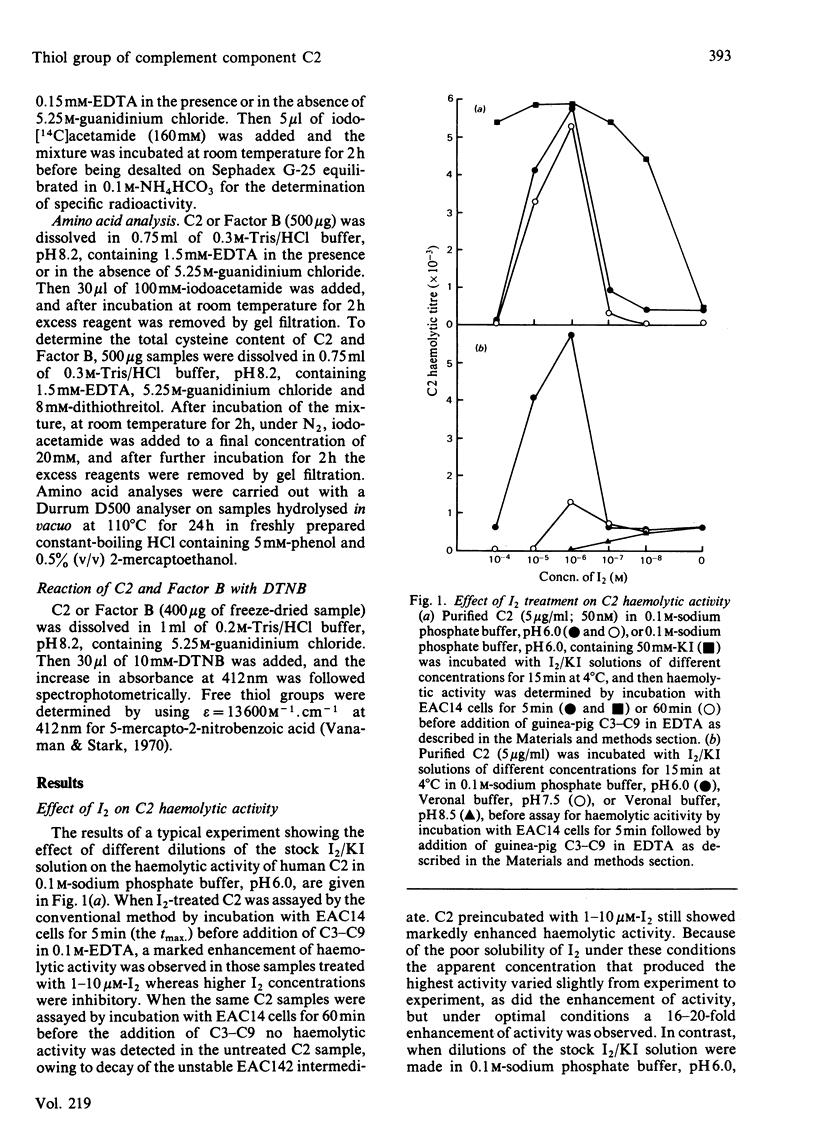
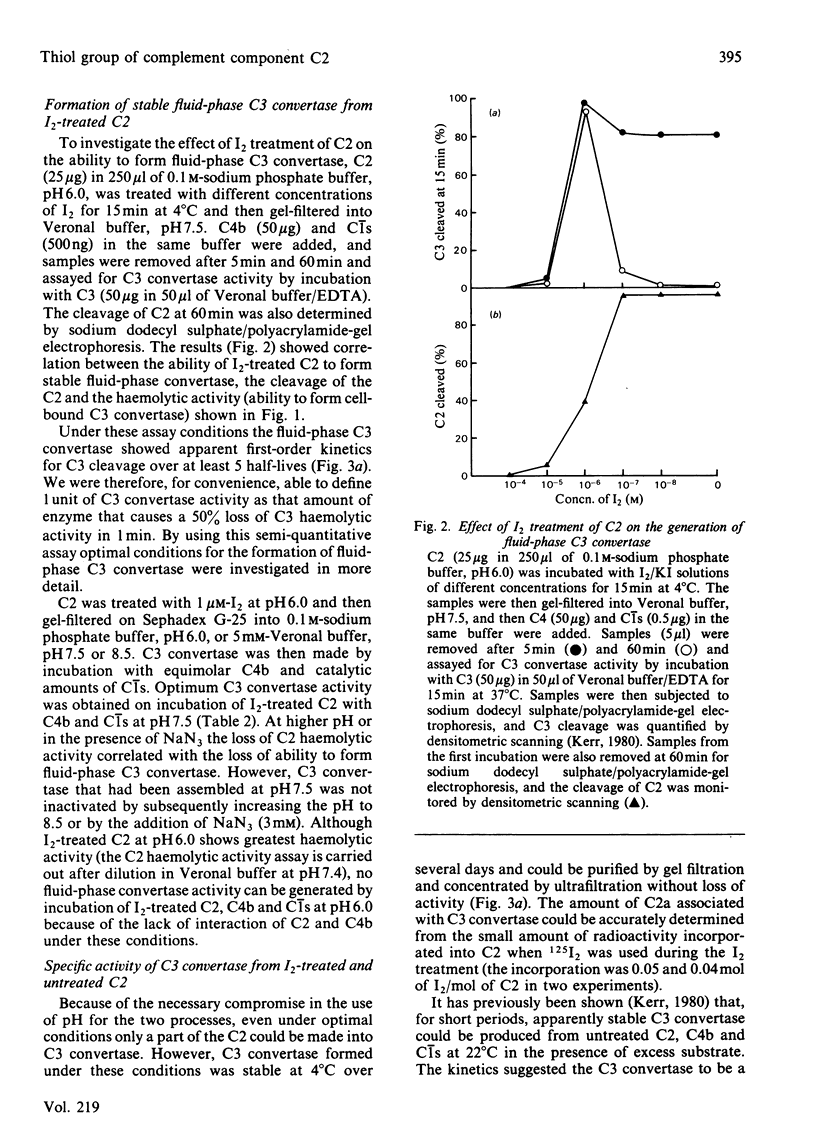
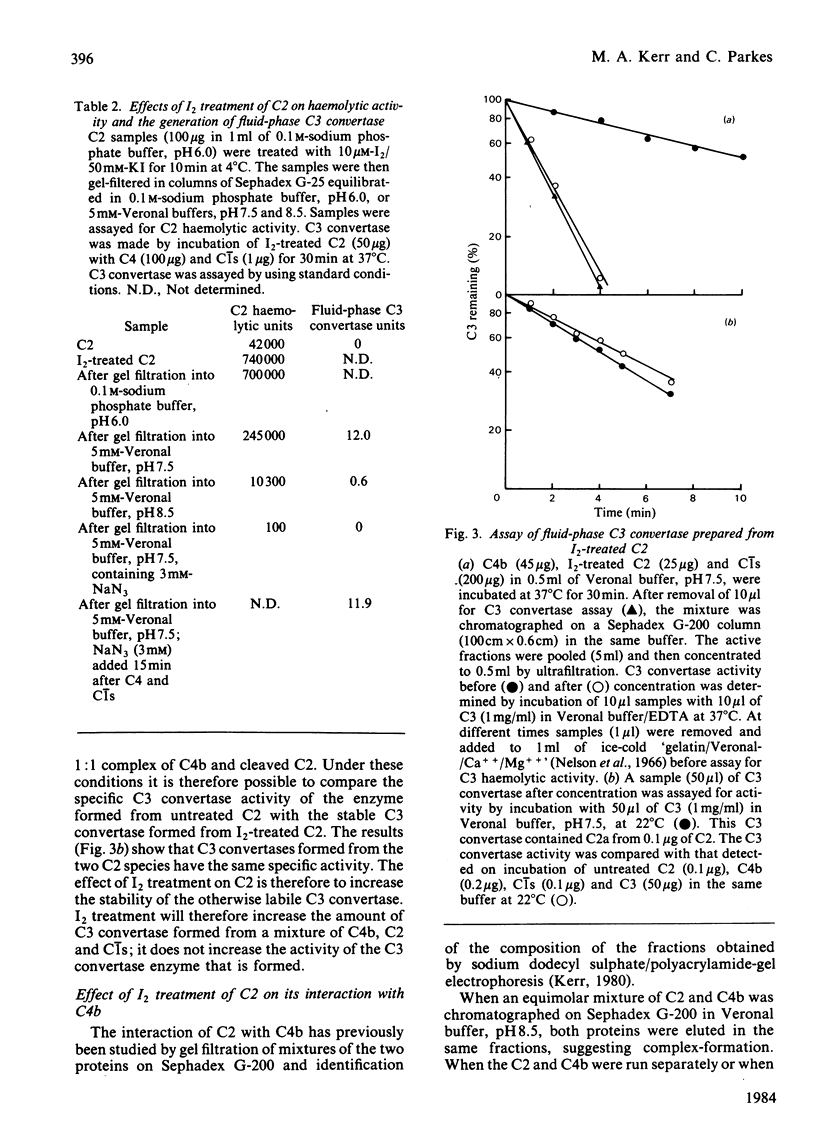
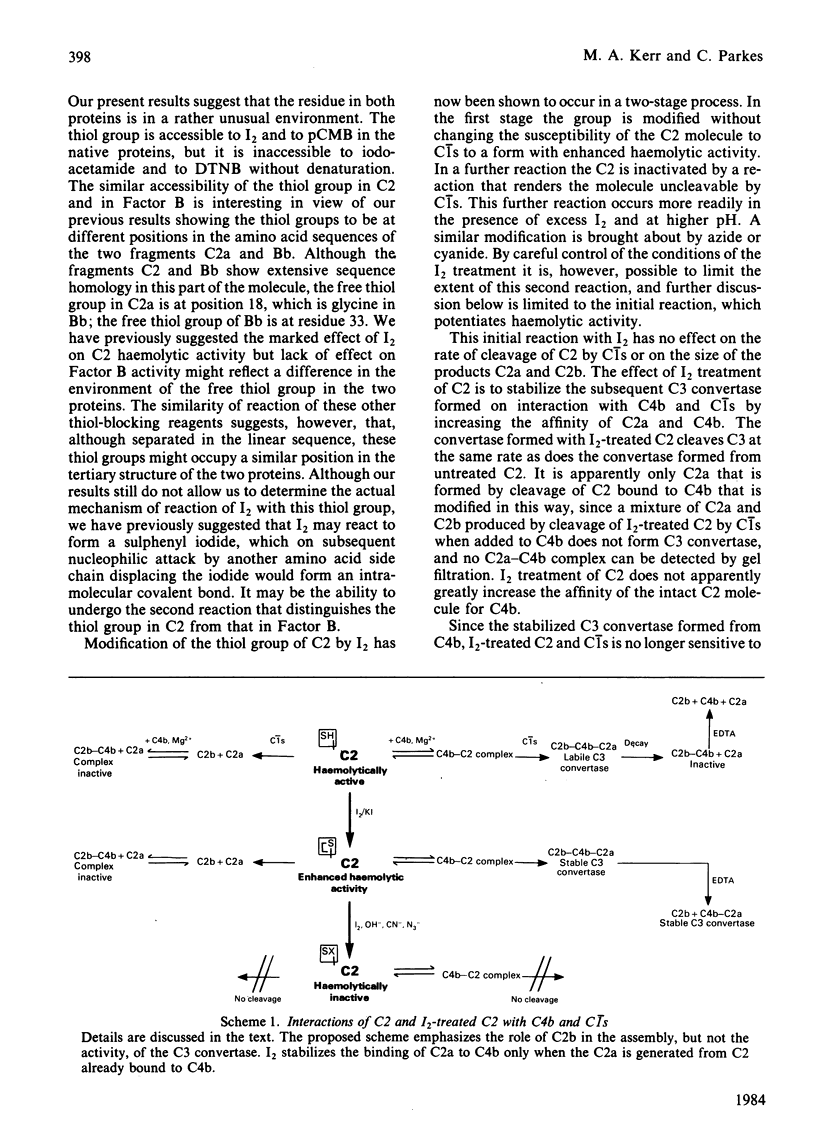
I2 can react with complement component C2 in a two-stage process. In the first stage, a form of C2 with enhanced haemolytic activity is produced. This form of C2 is cleaved to C2a and C2b by C1s at the same rate as native C2. The enhanced C2 haemolytic activity correlates with the ability to form a stable fluid-phase C3 convertase on addition of the C2 to C4b and C1s. It reflects an increased affinity for C4b of C2a formed from I2-treated C2, although the affinity for C4b of I2-treated C2 itself is not markedly increased. The specific activity of C3 convertase formed from I2-treated C2 is the same as that formed from native C2. The second stage of the reaction with I2, which is favoured at high pH or in the presence of excess I2, inactivates C2 on production of a species that cannot be cleaved by C1s. The presence of a single free thiol group in C2, which is the site of modification by I2, was confirmed by titration with p-chloromercuribenzoate, iodoacetamide and 5,5'-dithiobis-(2-nitrobenzoic acid). A single thiol group is also present in Factor B, and the cysteine residue, like that in C2, requires denaturation of the protein before reaction with iodoacetamide and 5,5'-dithiobis-(2-nitrobenzoic acid) but not p-chloro- mercuribenzoate .
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christie D. L., Gagnon J., Porter R. R. Partial sequence of human complement component factor B: novel type of serine protease. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4923–4927. doi: 10.1073/pnas.77.8.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N. R., Polley M. J., Müller-Eberhard H. J. The second component of human complement (C2): quantitative molecular analysis of its reactions in immune hemolysis. Immunochemistry. 1970 Apr;7(4):341–356. doi: 10.1016/0019-2791(70)90237-5. [DOI] [PubMed] [Google Scholar]
- Gigli I., Porter R. R., Sim R. B. The unactivated form of the first component of human complement, C1. Biochem J. 1976 Sep 1;157(3):541–548. doi: 10.1042/bj1570541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr M. A., Gagnon J. The purification and properties of the second component of guinea-pig complement. Biochem J. 1982 Jul 1;205(1):59–67. doi: 10.1042/bj2050059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr M. A. Human factor B. Methods Enzymol. 1981;80(Pt 100):102–112. doi: 10.1016/s0076-6879(81)80010-9. [DOI] [PubMed] [Google Scholar]
- Kerr M. A., Porter R. R. The purification and properties of the second component of human complement. Biochem J. 1978 Apr 1;171(1):99–107. doi: 10.1042/bj1710099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr M. A. The human complement system: assembly of the classical pathway C3 convertase. Biochem J. 1980 Jul 1;189(1):173–181. doi: 10.1042/bj1890173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesavre P. H., Hugli T. E., Esser A. F., Müller-Eberhard H. J. The alternative pathway C3/C5 convertase: chemical basis of factor B activation. J Immunol. 1979 Aug;123(2):529–534. [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Schreiber R. D. Molecular biology and chemistry of the alternative pathway of complement. Adv Immunol. 1980;29:1–53. doi: 10.1016/s0065-2776(08)60042-5. [DOI] [PubMed] [Google Scholar]
- Nagasawa S., Stroud R. M. Cleavage of C2 by C1s into the antigenically distinct fragments C2a and C2b: demonstration of binding of C2b to C4b. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2998–3001. doi: 10.1073/pnas.74.7.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- Parkes C., Gagnon J., Kerr M. A. The reaction of iodine and thiol-blocking reagents with human complement components C2 and factor B. Purification and N-terminal amino acid sequence of a peptide from C2a containing a free thiol group. Biochem J. 1983 Jul 1;213(1):201–209. doi: 10.1042/bj2130201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley M. J., Müller-Eberhard H. J. Enharncement of the hemolytic activity of the second component of human complement by oxidation. J Exp Med. 1967 Dec 1;126(6):1013–1025. doi: 10.1084/jem.126.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B., Porter R. R. The proteolytic activation systems of complement. Annu Rev Biochem. 1981;50:433–464. doi: 10.1146/annurev.bi.50.070181.002245. [DOI] [PubMed] [Google Scholar]
- Vanaman T. C., Stark G. R. A study of the sulfhydryl groups of the catalytic subunit of Escherichia coli aspartate transcarbamylase. The use of enzyme--5-thio-2-nitrobenzoate mixed disulfides as intermediates in modifying enzyme sulfhydryl groups. J Biol Chem. 1970 Jul 25;245(14):3565–3573. [PubMed] [Google Scholar]


