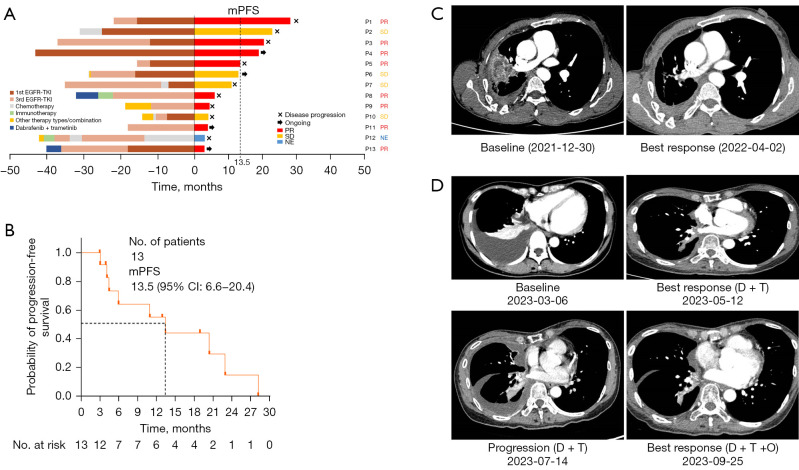
Figure 1.
Efficacy and patient cases of triple-targeted therapy. (A) Swimmer plot. The x-axis at 0 represents the start of triple-targeted therapy. The left side of the y-axis indicates the treatment timelines before triple-targeted therapy, and the right side of the y-axis represents the treatment assessment after triple-targeted therapy. (B) Kaplan-Meier survival curve for progression-free survival. (C) Patient case—thoracic lesion changes before and after triple-targeted therapy. Patient No. 5 achieved a partial response after triple-targeted therapy. The thoracic lesions exhibited a significant reduction (75%) following the therapy. The triple-targeted therapy maintained its efficacy for a duration of 15 months. (D) Patient case—changes of thoracic lesions at baseline, after dual therapy, at progression, and after triple-targeted therapy. Patient No. 13 initially received dabrafenib and trametinib after experiencing progression on EGFR-TKI therapy upon the emergence of a BRAF V600E mutation. Although a partial response was initially achieved with the dual therapy, the disease progressed after 3 months. Subsequently, triple-targeted therapy was started, resulting in a PR after 2 months. mPFS, median progression-free survival; PR, partial response; SD, stable disease; NE, non-evaluable; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor; 1st EGFR-TKI, first generation EGFR-TKI; 3rd EGFR-TKI, third generation EGFR-TKI; BRAF, B-Raf proto-oncogene, serine/threonine kinase; D + T, dabrafenib + trametinib; D + T + O, dabrafenib, trametinib and osimertinib.