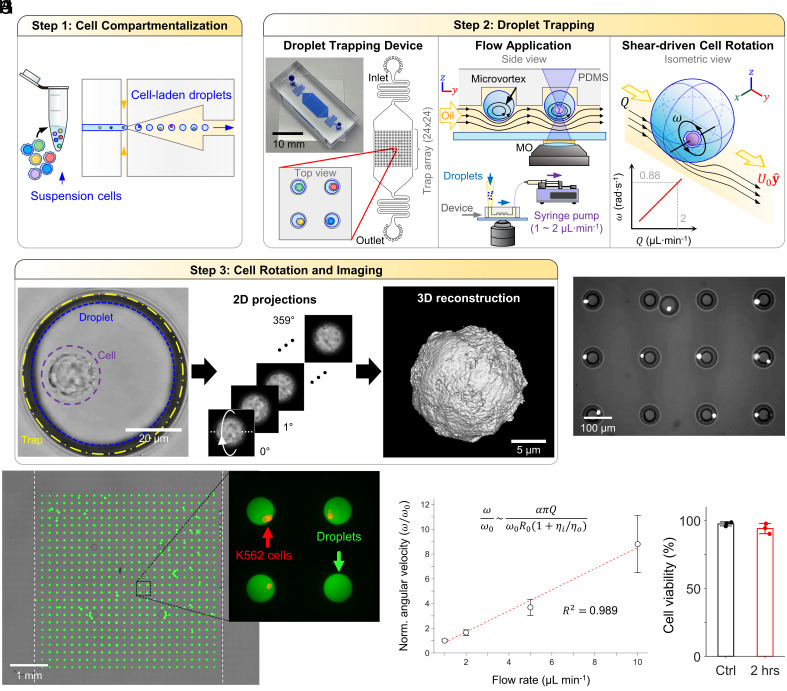
Fig. 1.
ADOPT Workflow and operation. (A) A structurally heterogeneous population of cells is first compartmentalized into water-in-oil droplets. (B) Cell-laden droplets are then loaded into a PDMS microfluidic trap array (24×24). Each trap consists of an inverted, circular microwell that can trap exactly one cell-laden droplet. Continuous oil perfusion transfers shear stress to the inside of droplets, generating recirculation microvortices that drive cell self-rotation. The application of flow rate (1 to 2 µL min−1) leads to linear increases in flow velocity (80 to 160 µm s−1) and shear forces at the droplet surface (2.1 to 4.2 mPa), which in turn linearly drive cell angular velocity (0.44 to 0.88 rad s−1). (C) Time-lapse frame acquisition of the rotating cells allows their full 360° observation, which can be used to approximate their 3D structure through point spread function (PSF)-informed OPT reconstruction algorithms. (D) Micrograph of multiple droplets containing rotating K562 cells stained with Hoechst 33342 dye. (E) Microfluidic trap array image obtained via image stitching, showcasing 100% loaded traps with 95% single droplet occupancy. Fluorescein dextran was used to enhance droplet visualization. (F) Blow-up of (E), displaying four droplets with stained suspension cells (K562, anti-CD45-PE). (G) Normalized angular velocity of encapsulated cells as a function of external oil flow rate. (H) K562 cell viability inside droplets when subjected to 2 h of continuous oil flow rate (1 µL min−1).