Abstract
4-Membered heterocycles have been increasingly exploited in medicinal chemistry and, as small polar motifs, often show important influence on activity and physicochemical properties. Thietane dioxides similarly offer potential in both agricultural and pharmaceutical applications but are notably understudied. Here we report a divergent approach to 3,3-disubstituted thietane dioxide derivatives by forming carbocations on the 4-membered ring with catalytic Lewis or Brønsted acids. Benzylic tertiary alcohols of the thietane dioxides are coupled directly with arenes, thiols, and alcohols.
Introduction
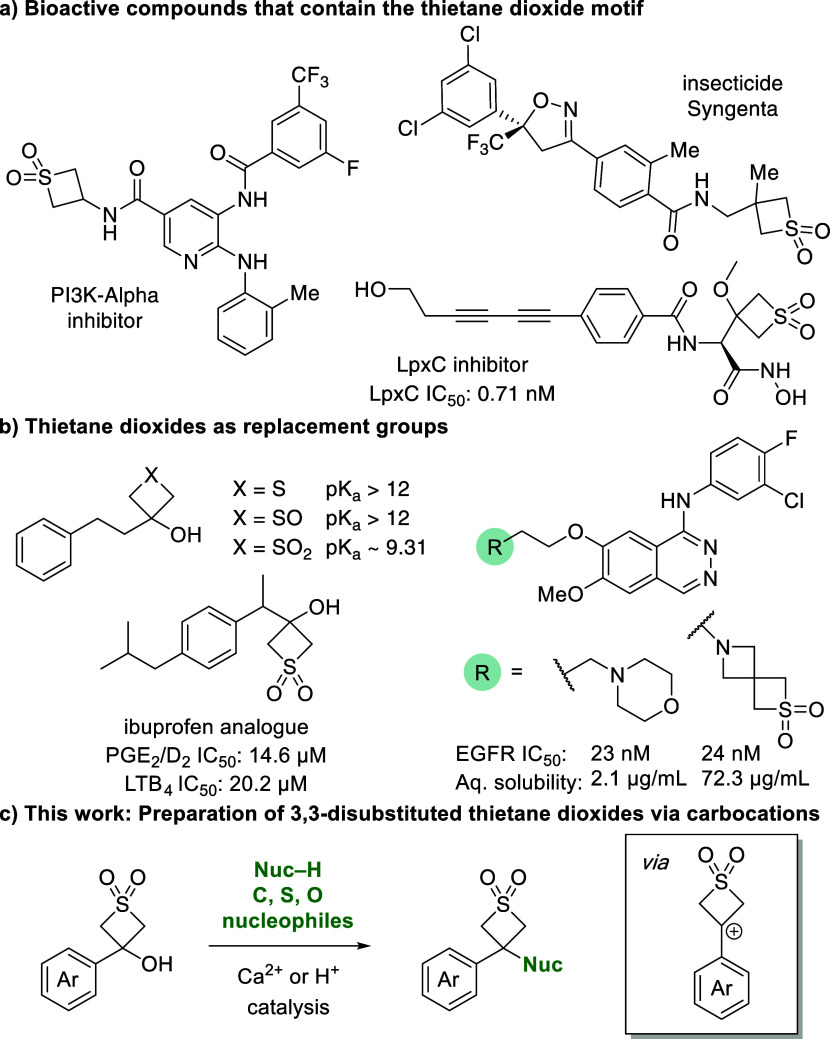
4-Membered heterocycles have been of notable interest in medicinal chemistry due to the potential to provide attractive polar and 3-dimensional motifs of low molecular weight and high H-bonding potential.1 Recent years have seen extensive development of the applications of oxetanes and azetidines.2 On the other hand, thietanes and their oxidized forms are much less studied and as such present interesting opportunities for development.3 Thietane dioxides in particular present interesting potential, being stable to further oxidation, that has been little exploited. The thietane dioxides may be considered expanded sulfones, though the oxygen atoms are rotated by 90° in the thietane dioxides, in plane with the substituents at the 3-position. Compounds bearing thietane dioxides have been reported in biologically active compounds in medicinal and agrochemistry (Figure 1). Recently a PI3K-Alpha inhibitor containing a thietane dioxide was reported as a potential cancer therapeutic.4 LpxC inhibitors containing thietane dioxides were disclosed as potential antibacterial agents, whereby a cocrystal with the enzyme displayed H-bonding with a lysine side chain, benefiting from the expanded size of the thietane dioxide compared to a methyl sulfone.5 Syngenta patented a series compounds containing pendant thietane dioxides as insecticides.6 Preliminary investigations have also studied thietane dioxide derivatives as replacements for carbonyl groups in carboxylic acids which maintained some acidity in comparison to oxetanols,7 including in ibuprofen analogues, and in spirocyclic morpholine analogues as solubilizing motifs (Figure 1b).8
Figure 1.
a) Examples of thietane dioxides in medicinal chemistry and agricultural applications. b) Thietane dioxides as replacement groups. c) This work: synthesis of 3,3-disubstituted thietane dioxide derivatives.
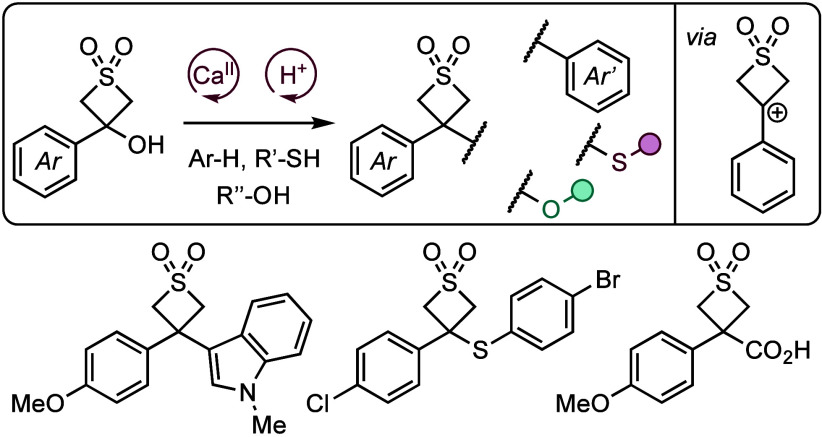
We have been interested in the synthesis of 3,3-disubstituted aryl-oxetanes and azetidines through the catalytic generation of carbocationic intermediates. The use of Lewis (Li+, Ca2+, and Fe3+ salts) and Brønsted acid catalysts has proven useful in selectively activating the 4-membered ring benzylic tertiary alcohols for Friedel–Crafts alkylation,9 and alkylation of thiols10 and alcohols.11,12 We envisaged that a similar approach may be viable on thietane dioxides, and as such provide a facile route to 3,3-disubstituted thietane dioxides, exploiting thietane-3-one as a readily available precursor.13 Here we report the development of a calcium-catalyzed reaction of 3-aryl-thietan-3-ol dioxides with arene and thiol nucleophiles, and a Brønsted acid catalyzed reaction with alcohols. This strategy provides arylthietane dioxide derivatives in a short divergent route expanding the available chemical space of 4-membered heterocycles. The 3,3-disubstituted products display high chemical stability and potential for further diversification.
Results and Discussion
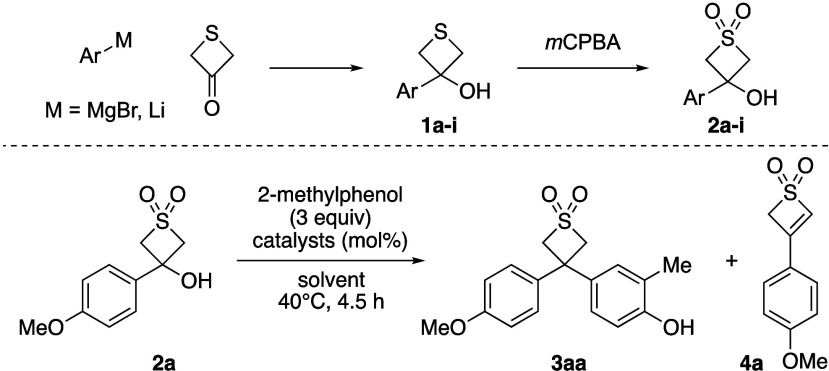
The study started from thietane-3-one, a readily available inexpensive precursor that reacts as a typical ketone with Grignard or organolithium reagents for the preparation of thietanols 1,14 which were readily converted to thietane dioxides 2 by oxidation with mCPBA (Table 1). Initial studies to generate the carbocation used thietanol dioxide 2a, which was readily prepared by the addition of commercial 4-methoxyphenylmagnesium bromide solution to thietane-3-one on >5 g scale. Based on our previous conditions with oxetanols and azetidinols,9a we then surveyed Lewis acids and Brønsted acids for the dehydrative generation of the benzylic carbocation on the thietane dioxide to trap with arene nucleophiles. Treating alcohol 2a with lithium salts in the presence of o-cresol under conditions successful for oxetanes formed the diarylthietane dioxide 3aa in low yield (Table 1 entry 1). A similar quantity of stable 3-aryl-2H-thiete 1,1-dioxide 4a was also formed, presumably through E1 elimination from the carbocation intermediate. On the other hand, Ca2+, Fe3+, and H+ catalysts all gave full conversion of the substrate and good yields of the diarylthietane dioxide product, but still with significant amounts of the elimination product 4a (entries 2–4). The solvent could be changed from dichloromethane to toluene as a more acceptable solvent for use on scale with similar results (entry 5, with CaII catalyst). A preliminary reaction scope was then examined using the Ca-catalyst due to ease of handling, but less reactive substrates required elevated temperatures to initiate a reaction. Therefore, we reexamined higher temperature conditions on the model substrate 2a. Pleasingly the thietane dioxide derivatives displayed full stability under elevated temperatures in toluene and moreover gave a notable increase in yield and decrease in formation of the thiete dioxide side product. Using 110 °C provided quantitative conversion, and a 93% isolated yield of 3aa (entry 7 and Scheme 1).
Table 1. Selected Optimization for Friedel–Crafts Reaction from Thietanol-Dioxide 2a and o-Cresol.
| yield
(%)b |
||||
|---|---|---|---|---|
| entrya | catalyst (mol %) | solvent | 3aa | 4a |
| 1 | Li(NTf2) (11) + nBu4NPF6 (5.5) | CH2Cl2 | 24 | 17 |
| 2 | FeCl3 (5) | CH2Cl2 | 74 | 12 |
| 3 | Ca(NTf2)2 (5) + nBu4NPF6 (5) | CH2Cl2 | 75 | 21 |
| 4 | HNTf2 (10) | CH2Cl2 | 82 | 18 |
| 5 | Ca(NTf2)2 (5) + nBu4NPF6 (5) | toluene | 63 | 37 |
| 6c | Ca(NTf2)2 (5) + nBu4NPF6 (5) | toluene | 72 | 23 |
| 7d | Ca(NTf2)2 (5) + nBu4NPF6 (5) | toluene | 97 | 0 |
Reactions on a 0.20 mmol scale.
Yields calculated by analysis of the 1H NMR spectrum of the crude reaction mixture using 1,3,5-trimethoxybenzene as internal standard.
Reaction run at 60 °C.
Reaction run at 110 °C.
Scheme 1. Reaction Scope with Arene, Thiol, and Alcohol Nucleophiles.
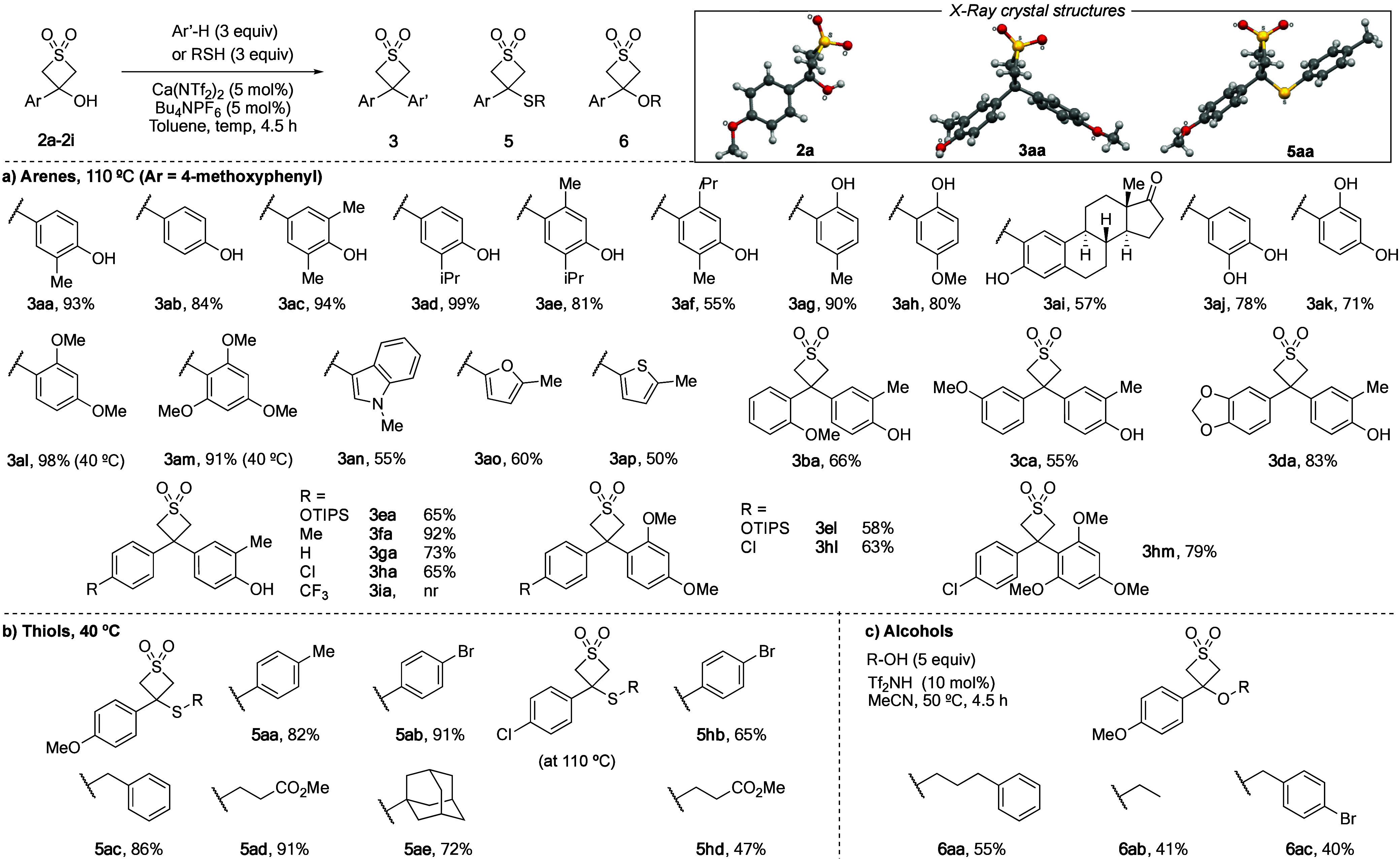
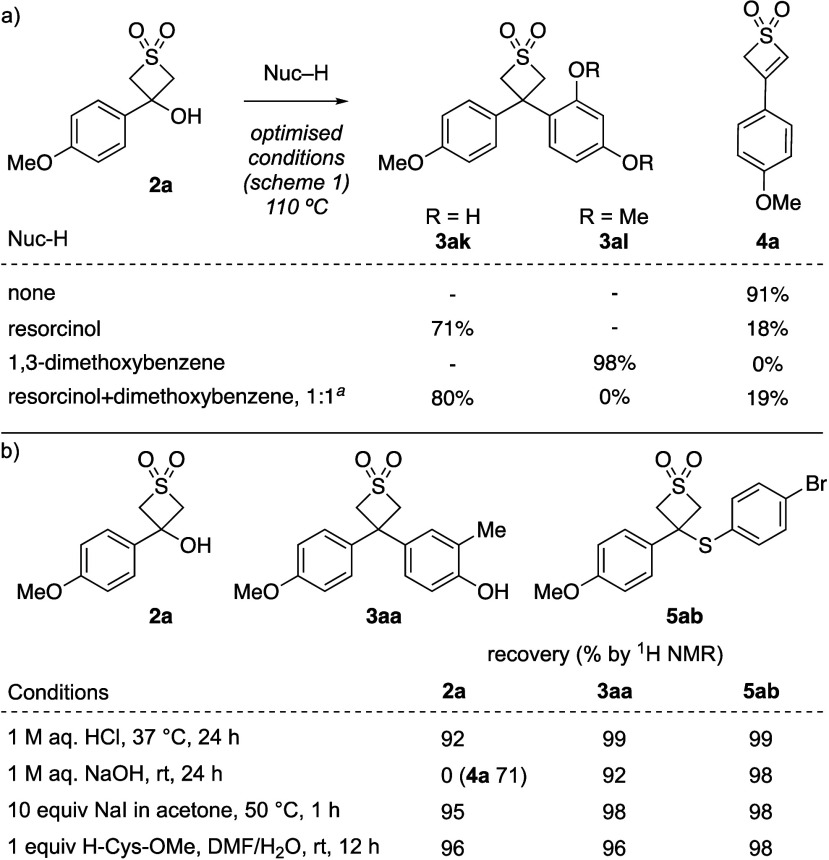
With high yielding conditions in hand, we examined the scope of the Friedel–Crafts reaction, through the variation of arene nucleophiles and thietane dioxide substrates (Scheme 1). Phenols were successful with complete C4 regioselectivity when that position was unsubstituted (3aa–3ak). Phenol itself gave diaryl thietane dioxide 3ab in 84% yield on a 1 mmol scale. Substituents at C3 were tolerated, including larger isopropyl groups, with little reduction in yield (3ae, 3af). 4-Substituted phenols were alkylated at C2 in high yields, including with cholesterol as a nucleophile (3ag–3ai). Catechol and resorcinol nucleophiles were also successful and yielded single regioisomers (3aj, 3ak). In contrast to previous observations with oxetanes,9a,9d there was no indication of opening of the thietane dioxide ring by ortho-hydroxyl groups. Nonphenolic arenes di- and trimethoxybenzene reacted effectively at 40 °C, with 98%, and 91% isolated yields (3al, 3am), without elimination to the thiete dioxide. Heterocycle nucleophiles N-methylindole, 2-methylfuran and 2-methylthiophene were also successful (3an–3ap). Electron-poor arenes like 4-bromophenol were unsuccessful, yielding only the elimination product 4a. Aniline nucleophiles were unsuccessful and returned unreacted 2a.
Varied substitution patterns were well tolerated on the arene of the thietanol dioxide (3ba–3hm). Methoxy substituents were tolerated in ortho- and meta-positions as well as a benzodioxole ring (3ba–3da). Substituents in the para position were also well tolerated, including an OTIPS group without any observed deprotection (3ea, 3el), electron neutral tolyl and phenyl derivatives (3fa, 3ga), and electron withdrawing 4-chlorophenyl derivative (3ha, 3hl). A para-CF3 derivative was unsuccessful, with recovered starting materials even under thermal activation up to 180 °C in dichlorobenzene. Attempts to extend the process to thietane and thietane oxide substrates were also unsuccessful, resulting in degradation, likely due to the transannular involvement of the sulfur lone pairs, which is not possible with the thietane dioxides. We propose thietane dioxides form a planar carbocation intermediate, analogous to that described for oxetanes.15
Next, we extended the reaction to thiol nucleophiles, to form 3-sulfanyl thietane dioxides (Scheme 1b). Both aromatic and aliphatic thiols were well tolerated in the alkylation with thietanol dioxide 2a, and the reaction proceeded smoothly at 40 °C (5aa–5ae). Alkylation using the less electron-rich thietanol dioxide 2h was also successful (5hb, 5hd) but required higher thermal activation (110 °C). Although the direct application of the reaction conditions to alcohol nucleophiles was unsuccessful, changing to Brønsted acid catalysis (Tf2NH, 10 mol %) in MeCN achieved the O-alkylation of primary and benzylic alcohols (6aa–6ac). Secondary alcohols were not tolerated due to a reversible C–O bond formation but irreversible elimination step funnelling the material to thiete dioxide 4a.
Several derivatives were further characterized by X-ray diffraction analysis of single crystals (2a, 3aa, 3ac, and 5aa, Scheme 1 boxed). Thietanol 2a showed a puckered thietane dioxide ring (29.4°) toward the hydroxyl group, suggestive of an intramolecular H-bond. On the other hand, diarylthietane dioxides were less puckered (3aa 14.0°; 3ac 16.9°) and the toluene sulfide derivative 5aa displayed a planar thietane dioxide ring (1° puckering angle). The dihedral conformation about the thietane-C–S bond is such that the tolyl group is aligned to the thietane S=O.
To better understand the effect of different nucleophiles and temperature on the reaction, a series of control experiments was performed. In the absence of a nucleophile, the thiete dioxide product formed through elimination was isolated in high yield (91%, Scheme 2a). Thiete dioxides have themselves have been demonstrated as suitable substrates for further reactions including cycloaddition, metalation and C–H functionalization.14 The effect of phenolic nucleophiles on elimination were investigated in a competition experiment. Both resorcinol and dimethoxybenzene undergo Friedel–Crafts alkylation with thietanol 2a in high yield, but 4a is formed only with resorcinol. A competition experiment with a 1:1 mixture of resorcinol and dimethoxybenzene gave only the phenolic diaryl product 3ak, but also formed thiete 4a, suggestive of a noninnocent role of the phenolic hydroxyl groups in the elimination process (Scheme 2a). Treating 2a with resorcinol alone does not result in elimination, suggesting a role as a basic site, perhaps via an O-linked intermediate.9b Resubmitting thiete 4a to the optimized reaction conditions with dimethoxybenzene gave only recovered 4a. On the other hand, treating 4a with resorcinol under the optimized conditions formed 3ak in a high 88% yield. Subjecting 3ak to the reaction conditions in the presence of dimethoxybenzene gave no reaction suggesting the reaction is not reversible. Together, this suggests a more complex role for the phenol nucleophiles to both promote the elimination pathway but also return the thiete dioxide to the catalytic cyclic through protonation. Indeed, reacting thiete 4a with o-cresol gave 3aa in low yield 37% (by 1H NMR). This explains the beneficial effect of the higher reaction temperature as the carbocation can be regenerated from the side product 4a in the presence of the acidic nucleophile.
Scheme 2. Competition experiments and stability studies.
yield by 1H NMR for competition experiment.
The chemical stability of 3,3-disubstituted thietane-1,1-dioxides was investigated by submitting thietan-3-ol 2a, diarylthietane dioxide 3aa, and sulfide 5ab to a range of conditions (Scheme 2b). In general, quantitative recovery of the substrates was observed across acidic (1 M HCl at 37 °C) and basic conditions (1 M NaOH), as well as in the presence of nucleophiles (NaI, and cysteine methyl ester). On treatment with aqueous 1 M NaOH, thietan-3-ol dioxide 2a degraded via elimination to thiete 4a.
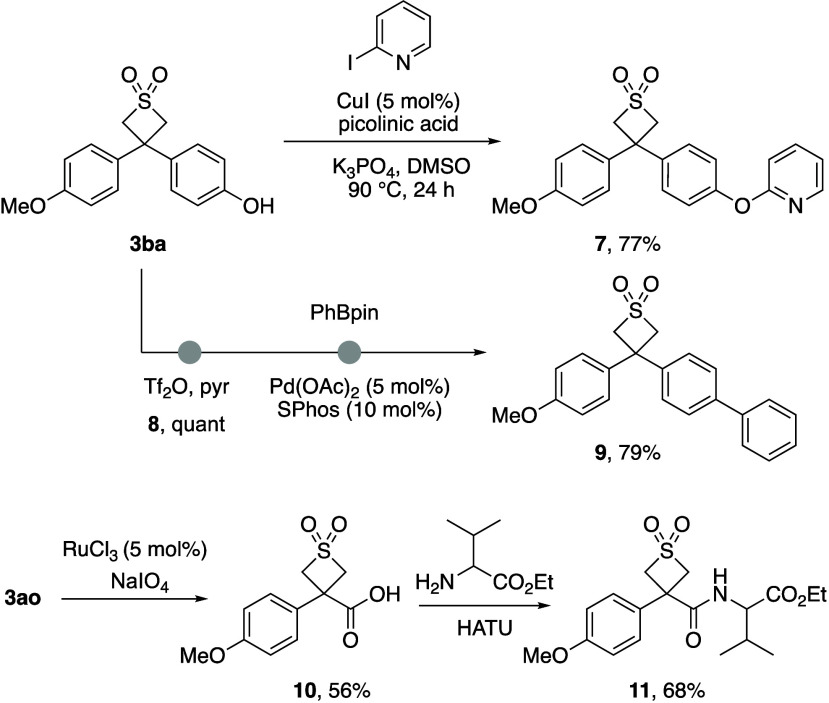
The phenolic functionality provides a handle for further functionalization through cross-coupling processes which was demonstrated with transition metal catalysis (Scheme 3). Ullmann arylation of 3ba with iodopyridine gave ether 7. Triflation was achieved in quantitative yield, which allowed for Suzuki–Miyaura coupling to form biaryl derivative 9. Carboxylic acid derivative 10 was prepared from furan 3ao by selective oxidative cleavage using ruthenium catalysis.16 The acid was readily applied in amide bond formation with standard conditions to give amide 11.
Scheme 3. Further functionalisation of 3,3-diarylthietane dioxides.
Overall, we present protocols for the preparation of 3,3-substituted thietane dioxides through the formation of carbocation intermediates. The application of increased temperatures was important to minimize formation of a thiete dioxide. We expect thietane dioxides to see broader application in medicinal chemistry with the development of new methods for their preparation, and the increase in commercial availability. This methodology provides a rapid and divergent approach to these disubstituted derivatives, and form C–C, C–S, and C–O bonds directly onto the intact 4-membered ring. The thietane dioxide rings display high chemical stability and are suitable for application in further cross-coupling and derivatization reactions.
Experimental Section
General Experimental Considerations
All nonaqueous reactions were run under an inert atmosphere (argon) with flame-dried or oven-dried glassware using standard techniques. Anhydrous solvents were obtained by filtration through drying columns (toluene, CH2Cl2) or used directly from commercial sources (MeCN) without drying. Reactions that required thermal activation were heated using a water bath (for temperatures up to 25 °C) or a silicone oil bath (for temperatures >25 °C). Flash column chromatography was performed using 230–400 mesh silica with the indicated solvent system according to standard techniques. Analytical thin-layer chromatography (TLC) was performed on precoated, glassbacked silica gel plates. Visualization of the developed chromatogram was performed by UV absorbance (254 nm) or aqueous potassium permanganate stains. Infrared spectra (νmax, FTIR ATR) were recorded in reciprocal centimeters (cm–1). Nuclear magnetic resonance spectra were recorded on 400 MHz spectrometers. Chemical shifts for 1H NMR spectra are recorded in parts per million from tetramethylsilane with the residual protic solvent resonance as the internal standard (chloroform: δ = 7.27 ppm, (CD3)2SO: δ = 2.50 ppm, CD3OD: δ = 3.31 ppm, acetone-d6: δ = 2.05 ppm). Data is reported as follows: chemical shift [multiplicity (s = singlet, d = doublet, t = triplet, quartet = q, pentet = p, m = multiplet and br = broad), coupling constant in Hz, integration, assignment]. 13C NMR spectra were recorded with complete proton decoupling. Chemical shifts are reported in parts per million from tetramethylsilane with the solvent resonance as the internal standard (13CDCl3: δ = 77.0 ppm, (13CD3)2SO: δ = 39.5 ppm, 13CD3OD: δ = 49.0 ppm, (13CD3)2O: δ = 29.8 ppm). J values are reported in Hz. Assignments of 1H/13C spectra were made by the analysis of δ/J values, and HSQC experiments as appropriate. 19F NMR spectra are indirectly referenced to CFCl3 automatically by direct measurement of the absolute frequency of the deuterium lock signal by the spectrometer hardware. Melting points were recorded using an Optimelt MPA100 apparatus and are uncorrected. The high-resolution mass spectrometry (HRMS) analyses were performed using electrospray ion source (ESI) or pneumatically assisted atmospheric pressure chemical ionization (APCI) using an atmospheric solids analysis probe (ASAP). ESI was performed using a Waters LCT Premier equipped with an ESI source operated in positive or negative ion mode. The software used was MassLynx 4.1. This software does not account for the electron and all the calibrations/references are calculated accordingly, i.e. [M + H]+ is detected and the mass is calibrated to output [M + H]. APCI was performed using an Orbitrap XL or Xevo G2S using an ASAP to insert samples into the APCI source. The sample was introduced at ambient temperature and the temperature increased until the sample vaporized. In mass spectrometry for thietan-3-ols, in some instances the ionization method fragmented the substrate to generate a carbocation, whereby [M – OH]+ was often found instead of [M + H]+.
Reagents: Commercial reagents were used as supplied or purified by standard techniques where necessary. Trifluoromethanesulfonimide (Tf2NH) was purchased from Fluorochem (CAS: 82113-65-3, product code: 093934), stored under argon in the fridge (+4 °C) and used without further purification. Calcium(II) bis(trifluoromethanesulfonimide) (Ca(NTf2)2) was purchased from Tokyo Chemical Industry (TCI) (CAS: 165324-09-4, product code: C3263), stored under argon in the desiccator. The concentration of n-BuLi (1.6 M in hexanes, purchased from Sigma-Aldrich, CAS: 109-72-8) was determined by titration with salicylaldehyde phenylhydrazone as an indicator before each reaction using a literature procedure.17 An average of three titrations was taken.
Synthesis of Thietanols from Thetan-3-one
3-(4-Methoxyphenyl)thietan-3-ol (1a)
4-Methoxyphenyl magnesium bromide (0.5 M in THF, 100 mL, 50.0 mmol, 1.1 equiv) was added dropwise to a solution of thietane-3-one (4.01 g, 45.5 mmol, 1.0 equiv) in THF (141 mL, 0.24 M) at −78 °C. After stirring at −78 °C for 30 min, the reaction mixture was warmed up to 25 °C and stirred for 1 h. The reaction was then quenched with sat. NH4Cl (80 mL). The mixture was extracted with CH2Cl2 (3 × 50 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated in vacuo using a rotary evaporator. Purification by flash column chromatography (5–10% EtOAc/pentane) afforded 3-(4-methoxyphenyl)thietan-3-ol 1a as yellow oil (5.40 g, 71%). Rf = 0.30 (25% EtOAc/pentane); IR (film)/cm–1 3401 (OH), 2994, 2935, 2833, 1610, 1580, 1511, 1462, 1441, 1362, 1301, 1249, 1211, 1178, 1108, 1032, 956, 830, 658, 579, 551; 1H NMR (400 MHz, CDCl3) δ 7.58 (d, J = 8.8 Hz, 2H, 2 × Ar–H), 6.94 (d, J = 8.8 Hz, 2H, 2 × Ar–H), 3.84 (s, 3H, OCH3), 3.65 (d, J = 10.4 Hz, 2H, CHH–S–CHH), 3.59 (d, J = 10.4 Hz, 2H, CHH–S–CHH), 2.77 (s, 1H, OH); 13C{1H} NMR (101 MHz, CDCl3) δ 159.3 (Ar–CqOMe), 136.7 (Ar–Cq), 125.6 (2 × Ar–C), 113.9 (2 × Ar–C), 78.9 (Cq), 55.3 (OCH3), 42.6 (CH2SCH2); HRMS (ESI) m/z calculated for C10H11OS [M – OH]+: 179.0531, found: 179.0536. The observed characterization data (IR, 1H and 13C NMR) were consistent with that previously reported.14
3-(2-Methoxyphenyl)thietan-3-ol (1b)
iPrMgCl·LiCl (1.30 M in THF, 2.54 mL, 3.3 mmol, 1.1 equiv) was added dropwise over 5 min to a solution of 2-iodoanisole (0.45 mL, 3.6 mmol, 1.2 equiv) in THF (4.0 mL) at 0 °C. The reaction mixture was stirred at 0 °C for a further 10 min and warmed to 25 °C for 2 h. A solution of thietanone (264 mg, 3.0 mmol, 1.0 equiv) in THF (6.0 mL) was added dropwise to the reaction mixture at −78 °C, then leave them to stir for 1 h. Following a further 24 h at 25 °C the reaction mixture was cooled to 0 °C and then quenched with sat. aq. NH4Cl (25 mL). The aqueous portion was extracted with Et2O (3 × 25 mL). The organic extracts were combined, dried over Na2SO4, filtered and concentrated under reduced pressure. Purification by flash chromatography (70% Et2O/pentane) afforded 3-(2-methoxyphenyl)thietan-3-ol 1b as yellow oil (412 mg, 70%). Rf = 0.31 (30% EtOAc/hexane); IR (film)/cm–1 3429 (OH), 2938, 2833, 1599, 1489, 1459, 1434, 1353, 1289,1233, 1177, 1020, 747, 577; 1H NMR (400 MHz, CDCl3) δ 7.46 (dd, J = 7.7, 1.7 Hz, 1H, Ar–H), 7.33 (td, J = 7.8, 1.6 Hz, 1H, Ar–H), 7.04 (td, J = 7.5, 1.1 Hz, 1H, Ar–H), 6.96 (d, J = 8.2 Hz, 1H, Ar–H), 4.24 (s, 1H, OH), 3.90 (s, 3H, OCH3), 3.66 (d, J = 10.1 Hz, 2H, CHH–S–CHH), 3.62 (d, J = 10.1 Hz, 2H, CHH–S–CHH); 13C{1H} NMR (101 MHz, CDCl3) δ 156.7 (Ar–Cq), 130.8 (Ar–Cq), 129.3 (Ar–C), 125.5 (Ar–C), 120.9 (Ar–C), 111.2 (Ar–C), 78.9 (Cq), 55.3 (O–CH3), 40.2 (2 × S–CH2).
3-(3-Methoxyphenyl)thietan-3-ol (1c)
3-Methoxyphenyl magnesium bromide (1.0 M in THF, 11 mL, 11 mmol, 1.1 equiv) was added dropwise to a solution of thietane-3-one (881 g, 10 mmol, 1.0 equiv) in THF (10 mL, 0.24 M) at −78 °C. After stirring at −78 °C for 30 min, the reaction mixture was warmed up to 25 °C and stirred for 3 h. The reaction was then quenched with sat. NH4Cl (50 mL). The mixture was extracted with CH2Cl2 (3 × 30 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated in vacuo using a rotary evaporator. Purification by flash column chromatography (10% EtOAc/pentane) afforded 3-(3-methoxyphenyl)thietan-3-ol 1c as yellow oil (1.14 mg, 58%). Rf = 0.18 (20% EtOAc/pentane); IR (film)/cm–1 3398 (OH), 2936, 2832, 1771, 1582, 1485, 1427, 1287, 1211, 1171, 1036, 964, 842, 781, 692, 564, 474; 1H NMR (400 MHz, CDCl3) δ 7.35 (d, J = 8.2 Hz, 1H, Ar–H), 7.26 (s, 1H, Ar–H), 7.20 (t, J = 2.2 Hz, 1H, Ar–H), 6.88 (dd, J = 8.2, 2.2 Hz, 1H, Ar–H), 3.84 (s, 3H, OCH3), 3.65 (d, J = 10.1 Hz, 2H, CHH–S–CHH), 3.56 (d, J = 10.1 Hz, 2H, CHH–S–CHH); 13C{1H} NMR (101 MHz, CDCl3) δ 159.9 (Ar–CqOMe), 146.2 (Ar–Cq), 129.8 (Ar–C), 116.5 (Ar–C), 113.3 (Ar–C), 110.2 (Ar–C), 79.1 (Cq), 55.4 (OCH3), 42.4 (2 × CH2–SO2); HRMS (APCI) m/z calculated for C10H11OS [M – OH]+: 179.0525, Found: 179.0527.
3-(Benzo[d][1,3]dioxol-5-yl)thietan-3-ol (1d)
iPrMgCl·LiCl (1.30 M in THF, 4.23 mL, 5.5 mmol, 1.1 equiv) was added dropwise over 5 min to a solution of 5-iodo-1,3-benzodioxole (1.49 g, 6.0 mmol, 1.2 equiv) in THF (4.0 mL) at 0 °C. The reaction mixture was stirred at 0 °C for a further 10 min and warmed to 25 °C for 2 h. A solution of thietanone (411 mg, 5.0 mmol, 1.0 equiv) in THF (6.0 mL) was added dropwise to the reaction mixture at −78 °C, then leave them to stir for 1 h. Following a further 24 h at 25 °C the reaction mixture was cooled to 0 °C and then quenched with sat. aq. NH4Cl (25 mL). The aqueous portion was extracted with Et2O (3 × 25 mL). The organic extracts were combined, dried over Na2SO4, filtered and concentrated under reduced pressure. Purification by flash chromatography (70% Et2O/pentane) afforded 3-(benzo[d][1,3]dioxol-5-yl)thietan-3-ol 1d as yellow oil (410 mg, 39%). Rf = 0.32 (30% EtOAc/hexane); IR (film)/cm–1 3370 (OH), 2937, 2889, 1484, 1435, 1233, 1171, 1031, 930, 860, 807, 561, 471; 1H NMR (400 MHz, CDCl3) δ 7.14 (d, J = 1.9 Hz, 1H, Ar–H), 7.11 (dd, J = 8.1, 1.9 Hz, 1H, Ar–H), 6.82 (d, J = 8.1 Hz, 1H, Ar–H), 5.97 (s, 2H, O–CH2–O), 3.61 (d, J = 10.5 Hz, 2H, CHH–S–CHH), 3.54 (d, J = 10.4 Hz, 2H, CHH–S–CHH); 13C{1H} NMR (101 MHz, CDCl3) δ 148.2 (Ar–Cq), 147.5 (Ar–Cq), 138.9 (Ar–Cq), 117.9 (Ar–C), 108.3 (Ar–C), 105.6 (Ar–C), 101.5 (S–CH2), 79.4 (Cq), 42.9 (O–CH2); HRMS (APCI) m/z Calculated for C10H11O3S [M + H]+: 211.0423; Found: 211.0422.
3-(4-((Triisopropylsilyl)oxy)phenyl)thietan-3-ol (1e)
iPrMgCl·LiCl (1.30 M in THF, 2.54 mL, 3.3 mmol, 1.1 equiv) was added dropwise over 5 min to a solution of (4-iodophenoxy)triisopropylsilane (0.45 mL, 3.6 mmol, 1.2 equiv) in THF (4.0 mL) at 0 °C. The reaction mixture was stirred at 0 °C for a further 10 min and warmed to 25 °C for 2 h. A solution of thietan-3-one (411 mg, 3.0 mmol, 1.0 equiv) in THF (6.0 mL) was added dropwise to the reaction mixture at −78 °C, then leave them to stir for 1 h. Following a further 24 h at 25 °C the reaction mixture was cooled to 0 °C and then quenched with sat. aq. NH4Cl (25 mL). The aqueous portion was extracted with Et2O (3 × 25 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated under reduced pressure. Purification by flash chromatography (70% Et2O/pentane) afforded 3-(4-((triisopropylsilyl)oxy)phenyl)thietan-3-ol 1e as yellow oil (122 mg, 12%). Rf = 0.36 (30% EtOAc/hexane); IR (film)/cm–1 3380 (OH), 2941, 2865, 1605, 1509, 1462, 1263, 1172, 1058, 1012, 995, 910, 881, 835, 682, 655, 554; 1H NMR (400 MHz, CDCl3) δ 7.50 (d, J = 8.7 Hz, 2H, 2 × Ar–H), 6.91 (d, J = 8.7 Hz, 2H, 2 × Ar–H), 3.62 (d, J = 9.8 Hz, 2H, CHH–S–CHH), 3.57 (d, J = 9.8 Hz, 2H, CHH–S–CHH), 1.28 (q, J = 7.0 Hz, 3H, 3 × Si–CH), 1.12 (d, J = 7.0 Hz, 18H, 6 × CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 155.8 (Ar–Cq), 137.2 (Ar–Cq), 125.5 (2 × Ar–C), 119.8 (2 × Ar–C), 78.9 (Cq), 42.6 (CH2–S–CH2), 17.9 (6 × CH3), 12.7 (3 × Si–C); HRMS (APCI) m/z Calculated for C18H29O2SSi [M – H]+: 337.1652; Found: 337.1662.
3-Hydroxy-3-(p-tolyl)thietane (1f)
4-Methylphenyl magnesium bromide (0.45 M in Et2O, 29 mL, 13 mmol, 1.3 equiv) was added dropwise to a solution of thietan-3-one (882 mg, 10 mmol, 1 equiv) in anhydrous THF (20 mL, 0.5 M) at −78 °C in a 100 mL round-bottom flask. The reaction mixture was stirred at −78 °C for 30 min, warmed to 25 °C and stirred for further 1 h. Sat. aq. NH4Cl (50 mL) was added and phases were separated. The aqueous phase was extracted with CH2Cl2 (3 × 50 mL). The organic layers were combined, washed with brine (100 mL), dried over Na2SO4, filtered and concentrated in vacuo using a rotatory evaporator. Purification by flash chromatography (20% Et2O/pentane) afforded 3-hydroxy-3-(p-tolyl)thietane 1f as a pale-yellow oil (1.36 g, 75%). Rf = 0.26 (30% Et2O/pentane); IR (film)/cm–1 3370 (OH), 2982, 2936, 1908, 1610, 1513, 1446, 1370, 1267, 1208, 1173, 1111, 1051, 948, 880, 820; 1H NMR (400 MHz, CDCl3) δ 7.56–7.54 (2 H, m, 2 × Ar–H), 7.25–7.23 (2 H, m, 2 × Ar–H), 3.65 (2 H, d, J = 10.4 Hz, CHH–S–CHH), 3.59 (2 H, d, J = 10.4 Hz, CHH–S–CHH), 2.81 (1 H, s, OH), 2.38 (3 H, s, CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 141.5 (Ar–Cq), 137.8 (Ar–Cq), 129.3 (2 × Ar–C), 124.2 (2 × Ar–C), 79.0 (Cq), 42.5 (CH2–S–CH2), 21.1 (CH3); HRMS (EI) m/z Calculated for C10H12OS.+ [M].+: 180.0603, Found: 180.0599.
3-Phenylthietan-3-ol (1g)
Phenyl magnesium bromide (1.0 M in THF, 50 mL, 50.0 mmol, 1.1 equiv) was added dropwise to a solution of thietane-3-one (4.01 g, 45.5 mmol, 1.0 equiv) in THF (141 mL, 0.24 M) at −78 °C. After stirring at −78 °C for 30 min, the reaction mixture was warmed up to 25 °C and stirred for 1 h. The reaction was then quenched with sat. NH4Cl (80 mL). The mixture was extracted with CH2Cl2 (3 × 50 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated in vacuo using a rotary evaporator. Purification by flash column chromatography (5–10% EtOAc/pentane) afforded 3-phenylthietan-3-ol 1g as yellow oil (5.19 g, 68%). Rf = 0.42 (20% EtOAc/pentane); IR (film)/cm–1 3369 (OH), 3057, 2937, 1493, 1447, 1361, 1210, 1174, 1052, 1028, 954, 913, 758, 693, 758; 1H NMR (400 MHz, CDCl3) δ 7.67 (dd, J = 7.6, 1.7 Hz, 2H, Ar–H), 7.43 (dd, J = 8.4, 6.7 Hz, 2H, Ar–H), 7.39–7.31 (m, 1H, Ar–H), 3.67 (d, J = 9.9 Hz, 2H, CHH–S–CHH), 3.59 (d, J = 10.1 Hz, 2H, CHH–S–CHH); 13C{1H} NMR (101 MHz, CDCl3) δ 144.4 (Ar–Cq), 128.6 (2 × Ar–C), 128.0 (Ar–C), 124.2 (2 × Ar–C), 79.0 (Cq), 42.4 (2 × CH2–S). HRMS (APCI) m/z Calculated for C9H10OS [M]+: 166.0447; Found: 166.0455. The observed characterization data (IR, 1H and 13C NMR) were consistent with that previously reported.14
3-(4-Chlorophenyl)thietan-3-ol (1h)
4-Chlorophenyl magnesium bromide (1.0 M in 2-methyl tetrahydrofuran, 11 mL, 11.0 mmol, 1.1 equiv) was added dropwise to a solution of thietane-3-one (881.3 g, 10.0 mmol, 1.0 equiv) in THF (29 mL, 0.25 M) at −78 °C. After stirring at −78 °C for 30 min, the reaction mixture was warmed up to 25 °C and stirred for 3 h. The reaction was then quenched with sat. NH4Cl (80 mL). The mixture was extracted with CH2Cl2 (3 × 50 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated in vacuo using a rotary evaporator. Purification by flash column chromatography (5–10% EtOAc/pentane) afforded 3-(4-chlorophenyl)thietan-3-ol 1h as yellow oil (1.09 g, 55%). Rf = 0.42 (20% EtOAc/pentane); IR (film)/cm–1 3366 (OH), 2938, 1595, 1489, 1398, 1210, 1090, 1052, 1010, 824, 543; 1H NMR (400 MHz, CDCl3) δ 7.59 (d, J = 8.6 Hz, 2H, 2 × Ar–H), 7.36 (d, J = 8.6 Hz, 2H, 2 × Ar–H), 3.55 (s, 4H, CHH–S–CHH); 13C{1H} NMR (101 MHz, CDCl3) δ 143.1 (Ar–Cq), 134.1 (Ar–Cq), 128.9 (2 × Ar–C), 126.0 (2 × Ar–C), 78.8 (Cq), 42.8 (2 × S–CH2). The observed characterization data (IR, 1H and 13C NMR) were consistent with that previously reported.18
3-(4-(Trifluoromethyl)phenyl)thietan-3-ol (1i)
iPrMgCl·LiCl (1.30 M in THF, 4.8 mL, 6.3 mmol, 1.05 equiv) was added dropwise over 5 min to a solution of 4-iodobenzotrifluoride (0.97 mL, 6.6 mmol, 1.1 equiv) in THF (7.0 mL) at 0 °C. The reaction mixture was stirred at 0 °C for a further 10 min and warmed to 25 °C for 3 h. A solution of thietanone (529 mg, 6.0 mmol, 1.0 equiv) in THF (13.0 mL) was added dropwise to the reaction mixture at 0 °C, Following a further 24 h at 25 °C. The reaction mixture was cooled to 0 °C and then quenched with sat. aq. NH4Cl (25 mL). The aqueous portion was extracted with Et2O (3 × 25 mL). The organic extracts were combined, dried over Na2SO4, filtered and concentrated under reduced pressure. Purification by flash chromatography (10% EtOAc/pentane) afforded 3-(4-(trifluoromethyl)phenyl)thietan-3-ol 1i as a yellow oil (952 mg, 68%). Rf = 0.55 (20% EtOAc/pentane); IR (film)/cm–1 3400 (OH), 2942, 1619, 1409, 1322, 1213, 1163, 1110, 1067, 1015, 955, 840, 702, 609, 472; 1H NMR (400 MHz, CDCl3) δ 7.84 (d, J = 8.1 Hz, 2H, Ar–H), 7.67 (d, J = 8.2 Hz, 2H, Ar–H), 3.61 (s, 4H, CHH–S–CHH); 13C{1H} NMR (101 MHz, CDCl3) δ 148.2 (Ar–Cq), 130.3 (q, J = 32.6 Hz, Ar–Cq), 125.6 (q, J = 3.8 Hz, 2 × Ar–C), 124.0 (q, J = 271 Hz, CF3), 124.7 (2 × Ar–C), 78.6 (Cq), 42.6 (2 × S–CH2); 19F NMR (377 MHz, CDCl3) δ −62.6; HRMS (APCI) m/z Calculated for C10H8SOF3 [M – H]−: 233.0253; Found: 233.0242.
Synthesis of Thietanol Dioxides by mCPBA Oxidation: General procedure A
m-CPBA (3.0 equiv) was added portionwise to a solution of thietan-3-ol (1.0 equiv) in CH2Cl2 (0.13 M) at 0 °C. After stirring at 0 °C for 5 min, the reaction mixture was warmed to 25 °C and stirred for 3.5 h. The reaction was then quenched with sat. aq. NaHCO3 (50 mL) followed by 50 mL CH2Cl2. The phases were separated and the organic layer was further washed with NaHCO3 (20 mL). The aqueous layer was extracted with CH2Cl2 (2 × 50 mL). The organic layers were combined, dried over Na2SO4, filtered and concentrated in vacuo. Purification by flash column chromatography afforded the thietan-3-ol dioxide.
3-Hydroxy-3-(4-methoxyphenyl)thietane 1,1-dioxide (2a)
Performed using general procedure A with thietanol 1a (1.05 g, 5 mmol) and m-CPBA (77%, 3.36 g, 15.0 mmol). Purification by flash column chromatography (20–30% acetone/pentane) afforded 3-hydroxy-3-(4-methoxyphenyl)thietane 1,1-dioxide 2a as a white solid (296 mg, 80%). Rf = 0.16 (30% acetone/hexane); mp = 127–129 °C; IR (film)/cm–1 3486 (OH), 3024, 2955, 2913, 2840, 1610, 1512, 1466, 1416, 1376, 1291, 1253, 1209, 1179, 1132, 1111, 1033, 1010, 964, 894, 827, 748, 646, 601, 550, 486, 475, 424; 1H NMR (400 MHz, CDCl3) δ 7.42 (d, J = 8.8 Hz, 2H, 2 × Ar–H), 6.96 (d, J = 8.8 Hz, 2H, 2 × Ar–H), 4.63 (d, J = 14.9 Hz, 2H, CHH–S–CHH), 4.42 (d, J = 14.9 Hz, 2H, CHH–S–CHH), 3.84 (s, 3H, OCH3); 13C{1H} NMR (101 MHz, CDCl3) δ 159.9 (Ar–CqOMe), 133.1 (Ar–CqCq), 126.2 (2 × Ar–CH), 114.4 (2 × Ar–CH), 78.2 (CH2SO2CH2), 64.6 (Cq), 55.4 (OCH3); HRMS (EI) m/z calculated for C10H12O4S.+ [M].+: 228.0451, Found: 228.0447.
3-Hydroxy-3-(2-methoxyphenyl)thietane 1,1-dioxide (2b)
Performed using general procedure A with thietanol 1b (196 mg, 1.0 mmol) and m-CPBA (77%, 672 mg, 3.0 mmol). Purification by flash column chromatography (30% acetone/pentane) afforded 3-hydroxy-3-(2-methoxyphenyl)thietane 1,1-dioxide 2b a white solid (226 mg, 99%). Rf = 0.36 (30% acetone/pentane); mp = 165–168 °C; IR (film)/cm–1 3422 (OH), 3042, 2969, 1484, 1458, 1296, 1258, 1220, 1162, 1168, 1100, 1023, 752, 676, 544, 444, 424; 1H NMR (400 MHz, CDCl3) δ 7.44–7.29 (m, 2H, 2 × Ar–H), 7.10–6.89 (m, 2H, 2 × Ar–H), 4.75 (d, J = 15.0 Hz, 2H, CHH–SO2–CHH), 4.37 (d, J = 14.9 Hz, 2H, CHH–SO2–CHH), 3.94 (s, 3H, CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 156.3 (Ar–Cq), 130.5 (Ar–C), 128.2 (Ar–Cq), 125.9 (Ar–C), 121.1 (Ar–C), 111.3 (Ar–C), 76.3 (2 × C–SO2), 63.7 (Cq), 55.5 (CH3); HRMS (APCI) m/z Calculated for C10H11O4S [M – H]+: 227.0373; Found: 227.0373.
3-Hydroxy-3-(3-methoxyphenyl)thietane 1,1-dioxide (2c)
Performed using general procedure A with thietanol 1c (392 mg, 2.0 mmol) and m-CPBA (77%, 1.34 g, 6.0 mmol). Purification by flash column chromatography (20% acetone/pentane) afforded 3-hydroxy-3-(3-methoxyphenyl)thietane 1,1-dioxide 2c as yellow oil (373 mg, 82%); Rf = 0.30 (30% acetone/pentane); IR (film)/cm–1 3458 (OH), 2961, 1602, 1586, 1430, 1125, 1314 (S=O), 1291, 1205, 1158, 1037, 907, 725, 446; 1H NMR (400 MHz, acetone-d6) δ 7.37 (t, J = 8.2 Hz, 1H, Ar–H), 7.20 (m, 2H, 2 × Ar–H), 6.93 (dd, J = 8.2, 2.5 Hz, 1H, Ar–H), 5.75 (s, 1H, OH), 4.66 (d, J = 15.0 Hz, 2H, CHH–S–CHH), 4.45 (d, J = 15.0 Hz, 2H, CHH–S–CHH), 3.84 (s, 3H, OCH3); 13C{1H} NMR (101 MHz, acetone-d6) δ 160.0 (Ar–Cq), 145.6 (Ar–Cq), 129.8 (Ar–C), 117.1 (Ar–C), 113.2 (Ar–C), 111.0 (Ar–C), 78.6 (2 × S–CH2), 63.1 (Cq), 54.8 (OCH3); HRMS (TOF-ES) m/z calculated for C12H15NO4SNa [M + MeCN + Na]+: 292.0622, Found: 292.0619.
3-(Benzo[d][1,3]dioxol-5-yl)-3-hydroxythietane 1,1-dioxide (2d)
Performed using general procedure A with thietanol 1d (252 mg, 1.2 mmol) and m-CPBA (77%, 864 mg, 3.6 mmol). Purification by flash column chromatography (30% acetone/pentane) afforded 3-(benzo[d][1,3]dioxol-5-yl)-3-hydroxythietane 1,1-dioxide 2d a white solid (293 mg, 83%). Rf = 0.23 (30% acetone/pentane); mp = 140–145 °C; IR (film)/cm–1 3438 (OH), 3039, 2973, 2905, 1685, 1487, 1438, 1291, 1177, 1150, 1031, 985, 918, 764, 624; 1H NMR (400 MHz, acetone-d6) δ 7.10–7.04 (m, 2H, 2 × Ar–H), 6.83 (d, J = 8.8 Hz, 1H, Ar–H), 5.99 (s, 2H, O–CH2–O), 5.66 (s, 1H, OH), 4.57 (d, J = 15.1 Hz, 2H, CHH–S–CHH), 4.36 (d, J = 15.1 Hz, 2H, CHH–S–CHH); 13C{1H} NMR (101 MHz, acetone-d6) δ 148.1 (Ar–Cq), 147.4 (Ar–Cq), 138.0 (Ar–Cq), 118.5 (Ar–C), 107.8 (Ar–C), 105.9 (Ar–C), 101.5 (OCH2), 78.4 (2 × S–CH2), 63.2 (Cq); HRMS(APCI) m/z Calculated for C10H9O5S [M – H]+: 241.0172; Found: 241.0165.
3-Hydroxy-3-(4-((triisopropylsilyl)oxy)phenyl)thietane 1,1-dioxide (2e)
Performed using general procedure A with thietanol 1e (336 mg, 1.0 mmol) and m-CPBA (77%, 672 mg, 3.0 mmol). Purification by flash column chromatography (30% acetone/pentane) afforded 3-hydroxy-3-(4-((triisopropylsilyl)oxy)phenyl)thietane 1,1-dioxide 2e as a white solid (296 mg, 80%). Rf = 0.16 (30% acetone/pentane); mp = 105–109 °C; IR (film)/cm–1 3460 (OH), 2944, 2866, 1607, 1511, 1267, 1210, 1167, 1128, 913, 839, 739, 685; 1H NMR (400 MHz, CDCl3) δ 7.33 (d, J = 8.7 Hz, 2H, 2 × Ar–H), 6.92 (d, J = 8.7 Hz, 2H, 2 × Ar–H), 4.63 (d, J = 14.9 Hz, 2H, CHH–SO2–CHH), 4.40 (d, J = 14.9 Hz, 2H, CHH–SO2–CHH), 1.26 (q, J = 7.3 Hz, 3H, 3 × Si–CH), 1.10 (d, J = 7.3 Hz, 18H, 6 × CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 156.6 (Ar–Cq), 133.4 (Ar–Cq), 126.1 (2 × Ar–C), 120.3 (2 × Ar–C), 78.3 (CH2–SO2–CH2), 64.6 (Cq), 17.9 (6 × CH3), 12.6 (3 × Si–CH); HRMS (APCI) m/z Calculated for C18H31O4SSi [M + H]+: 371.1707; Found: 371.1706.
3-Hydroxy-3-(p-tolyl)thietane 1,1-dioxide (2f)
Performed using general procedure A with thietanol 1f (180 mg, 1.0 mmol) and m-CPBA (77%, 672.4 mg, 3.0 mmol). Purification by flash column chromatography (30% acetone/pentane) afforded 3-hydroxy-3-(p-tolyl)thietane 1,1-dioxide 2f a white solid (177 mg, 83%). Rf = 0.36 (30% acetone/pentane); mp = 114–119 °C; IR (film)/cm–1 3455 (OH), 3027, 2958, 1515, 1383, 1307, 1206, 1165, 1126, 1040, 1008, 971, 818, 764, 595, 547, 483; 1H NMR (400 MHz, CDCl3) δ 7.37 (d, J = 8.0 Hz, 2H, Ar–H), 7.23 (d, J = 8.0 Hz, 2H, Ar–H), 4.59 (d, J = 15.1 Hz, 2H, CHH–S–CHH), 4.40 (d, J = 15.1 Hz, 2H, CHH–S–CHH), 2.37 (s, 3H, CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 138.7 (Ar–Cq), 138.5 (Ar–Cq), 129.7 (2 × Ar–C), 124.6 (2 × Ar–C), 78.4 (2 × S–CH2), 64.4 (Cq), 21.0 (CH3); HRMS (APCI) m/z Calculated for C10H10O3S [M – H]−: 211.0434; Found: 211.0428.
3-Hydroxy-3-phenylthietane 1,1-dioxide (2g)
Performed using general procedure A with thietanol 1g (333 mg, 2.0 mmol) and m-CPBA (77%, 1.34 g, 6.0 mmol). Purification by flash column chromatography (30% acetone/pentane) afforded 3-hydroxy-3-phenylthietane 1,1-dioxide as a 2g white solid (317 mg, 80%). Rf = 0.16 (30% acetone/pentane); mp = 103–109 °C; IR (film)/cm–1 3458 (OH), 3020, 2960, 1384, 1311, 1211, 1168, 1128, 1008, 972, 763, 699, 494; 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 7.0 Hz, 2H, 2 × Ar–H), 7.45 (t, J = 7.5 Hz, 2H, 2 × Ar–H), 7.39 (t, J = 7.2 Hz, 1H, Ar–H), 4.65 (d, J = 15.0 Hz, 2H, CHH–S–CHH), 4.43 (d, J = 15.0 Hz, 2H, CHH–S–CHH), 3.25 (s, 1H, OH); 13C{1H} NMR (101 MHz, CDCl3) δ 141.2 (Ar–Cq), 129.1 (2 × Ar–C), 128.8 (Ar–C), 124.7 (2 × Ar–C), 78.5 (2 × SO2–CH2), 64.7 (Cq); HRMS (APCI) m/z Calculated for C9H11O3S [M + H]+: 199.0423; Found: 199.0423.
3-(4-Chlorophenyl)-3-hydroxythietane 1,1-dioxide (2h)
Performed using general procedure A with thietanol 1h (401 mg, 2.0 mmol) and m-CPBA (77%, 1.34 g, 6.0 mmol). Purification by flash column chromatography (30% acetone/pentane) afforded 3-(4-chlorophenyl)-3-hydroxythietane 1,1-dioxide 2h a white solid (232.7 mg, 50%). Rf = 0.36 (30% acetone/pentane); mp = 168–175 °C; IR (film)/cm–1 3480 (OH), 3021, 1490, 1297,1214, 1177, 1132, 1096, 1011, 828, 754, 638, 543; 1H NMR (400 MHz, DMSO) δ 7.57 (d, J = 8.7 Hz, 2H, 2 × Ar–H), 7.48 (d, J = 8.6 Hz, 2H, 2 × Ar–H), 6.83 (s, 1H, OH), 4.68 (d, J = 15.3 Hz, 2H, CHH–S–CHH), 4.37 (d, J = 15.4 Hz, 2H, CHH–S–CHH); 13C{1H} NMR (101 MHz, DMSO) δ 143.0 (Ar–Cq), 132.4 (Ar–Cq), 128.3 (2 × Ar–C), 127.2 (2 × Ar–C), 78.3 (2 × CH2–S), 62.5 (Cq); HRMS (APCI) m/z Calculated for C9H10O3S35Cl [M + H]+: 233.0034; Found: 233.0034.
3-Hydroxy-3-(4-(trifluoromethyl)phenyl)thietane 1,1-dioxide (2i)
Performed using general procedure A with thietanol 1g (469 mg, 2.0 mmol) and m-CPBA (77%, 1.34 g, 6.0 mmol). Purification by flash column chromatography (20% acetone/pentane) afforded 3-hydroxy-3-(4-(trifluoromethyl)phenyl)thietane 1,1-dioxide 2i as a white solid (280.2 mg, 51%). Rf = 0.47 (20% acetone/pentane); mp = 138–141 °C; IR (film)/cm–1 3457 (OH), 3031, 2963, 1619, 1411, 1388, 1320 (S=O), 1212, 1166, 1111, 1068, 1014, 976, 843, 638, 513, 422; 1H NMR (400 MHz, acetone-d6) δ 7.90 (d, J = 8.1 Hz, 2H, 2 × Ar–H), 7.79 (d, J = 8.1 Hz, 2H, 2 × Ar–H), 6.04 (s, 1H, OH), 4.72 (d, J = 15.3 Hz, 2H, CHH–SO2–CHH), 4.50 (d, J = 15.3 Hz, 2H, CHH–SO2–CHH); 13C{1H} NMR (101 MHz, acetone-d6) δ 149.4 (Ar–Cq), 130.0 (q, J = 32.3 Hz, Ar–Cq), 126.8 (2 × Ar–C), 126.3 (q, J = 32.3 Hz, 2 × Ar–C) 126.3 (q, J = 272.5 Hz, CF3), 79.6 (2 × CH2–SO2), 63.8 (Cq); 19F NMR (377 MHz, acetone-d6) δ −63.1; HRMS (ESI) m/z Calculated for C10H8SO3F3 [M – H]−: 265.0152; Found: 265.0142.
Friedel–Crafts Reactions with Thietan-3-ol Dioxides: General Procedure B
Calcium(II) bis(trifluoromethanesulfonimide) (6.0 mg, 0.01 mmol, 0.05 equiv) and tetrabutylammonium hexafluorophosphate (4.0 mg, 0.01 mmol, 0.05 equiv) were added sequentially to a solution of thietane-3-ol dioxide (0.20 mmol, 1 equiv) and arene (0.60 mmol, 3 equiv) in toluene (0.4 mL, 0.5 M) in reaction vial. The reaction vial was sealed under argon, and the mixture was heated at 110 °C for 4.5 h then cooled to rt. Sat. aq. NaHCO3 (15 mL) was added followed by CH2Cl2 (15 mL). The phases were separated and the aqueous layer was extracted with CH2Cl2 (2 × 15 mL). The organic layers were combined, dried over Na2SO4, filtered and concentrated in vacuo. Purification by flash column chromatography afforded the diarylthietane dioxide.
3-(4-Hydroxy-3-methylphenyl)-3-(4-methoxyphenyl)thietane 1,1-dioxide (3aa)
Performed using general procedure B with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and 2-methylphenol (65.3 mg, 0.60 mmol, 3 equiv). Purification by flash column chromatography (3–5% Et2O/CH2Cl2) afforded diarylthietane dioxide 3aa as a white solid (59.2 mg, 93%). Rf = 0.18 (3% Et2O/CH2Cl2); IR (film)/cm–1 3432 (OH), 3024, 2957, 2929, 2837, 1607, 1509, 1460, 1413, 1396, 1305 (SO2), 1270, 1249, 1214, 1183, 1116, 1031, 909, 824, 771, 731, 600, 550, 486; 1H NMR (400 MHz, CDCl3) δ 7.19–7.17 (m, 2H, 2 × Ar–H), 6.99 (d, J = 2.6 Hz, 1H, Ar–H), 6.95–6.93 (dd, J = 8.3, 2.6 Hz, 1H, Ar–H), 6.89–6.86 (m, 2H, 2 × Ar–H), 6.72–6.69 (d, J = 8.3 Hz, 1H, Ar–H), 5.06 (s, 1H, OH), 4.86–4.83 (d, J = 12.9 Hz, 2H, CHH–SO2–CHH), 4.82–4.79 (d, J = 12.9 Hz, 2H, CHH–SO2–CHH), 3.80 (s, 3H, OCH3), 2.21 (s, 3H, CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 158.5 (Ar–CqO), 153.0 (Ar–CqO), 137.0 (Ar–Cq), 136.7 (Ar–Cq), 129.3 (Ar–C), 127.7 (2 × Ar–C), 125.2 (Ar–C), 124.5 (Ar–Cq), 115.1 (Ar–C), 114.2 (2 × Ar–C), 76.8 (CH2–SO2–CH2), 55.3 (OCH3), 36.4 (Cq), 16.0 (CH3); HRMS (APCI) m/z Calculated for C17H19O4S+ [M + H]+: 319.0999, Found: 319.1002.
3-(4-Hydroxyphenyl)-3-(4-methoxyphenyl)thietane 1,1-dioxide (3ab)
[1 mmol scale reaction] Calcium(II) bis(trifluoromethanesulfonimide) (30.0 mg, 0.05 mmol, 0.05 equiv) and tetrabutylammonium hexafluorophosphate (19.3 mg, 0.05 mmol, 0.05 equiv) were added sequentially to a solution of thietane dioxide 2a (228 mg, 1.0 mmol, 1 equiv) and phenol (282 mg, 3.0 mmol, 3.0 equiv) in toluene (2.0 mL, 0.5 M). The reaction mixture was stirred at 40 °C for 4.5 h then sat. aq. NaHCO3 (30 mL) was added followed by CH2Cl2 (30 mL). The phases were separated and the aqueous layer was extracted with CH2Cl2 (2 × 30 mL). The organic layers were combined, dried over Na2SO4, filtered and concentrated in vacuo using a rotatory evaporator. Purification by flash column chromatography (0–2% Et2O/CH2Cl2) afforded diarylthietane dioxide 3ab as a white solid (255 mg, 83%); mp = 186–188 °C; IR (film)/cm–1 3459 (OH), 2952, 1755, 1606, 1510, 1306, 1210, 1180, 1127, 1013, 831, 766, 644, 545; 1H NMR (400 MHz, CDCl3) δ 7.16 (d, J = 8.9 Hz, 2H, 2 × Ar–H), 7.12 (d, J = 8.7 Hz, 2H, 2 × Ar–H), 6.87 (d, J = 8.9 Hz, 2H, 2 × Ar–H), 6.79 (d, J = 8.7 Hz, 2H, 2 × Ar–H), 5.05 (s, 1H, OH), 4.82 (s, 4H, CHH–SO2–CHH), 3.79 (s, 3H, OCH3); 13C{1H} NMR (101 MHz, CDCl3) δ 158.6 (Ar–Cq), 154.7 (Ar–Cq), 137.0 (Ar–Cq), 136.7 (Ar–Cq), 128.0 (2 × Ar–C), 127.7 (2 × Ar–C), 115.7 (2 × Ar–C), 114.2 (2 × Ar–C), 77.0 (2 × CH2–SO2), 55.3 (OCH3), 36.4 (Cq); HRMS(ESI) m/z Calculated for C16H15O4S [M – H]−: 303.0697; Found: 303.0697.
3-(4-Hydroxy-3,5-dimethylphenyl)-3-(4-methoxyphenyl)thietane 1,1-dioxide (3ac)
Performed using general procedure B with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and 2,6-dimethylphenol (74.8 mg, 0.6 mmol, 3.0 equiv). Purification by flash column chromatography (0–2% Et2O/CH2Cl2) afforded diarylthietane dioxide 3ac as a white solid (62.5 mg, 94%). Rf = 0.38 (5% Et2O/CH2Cl2); mp = 186–188 °C; IR (film)/cm–1 3493, 3026, 2958, 2837, 1607, 1512, 1490, 1462, 1393, 1308 (SO2 st), 1253, 1216, 1183, 1129, 1030, 910, 833, 732; 1H NMR (400 MHz, CDCl3) δ 7.19 (d, J = 8.8 Hz, 2H, 2 × Ar–H), 6.87 (d, J = 8.8 Hz, 2H, 2 × Ar–H), 6.84 (s, 2H, 2 × Ar–H), 4.85 (d, J = 13.2 Hz, 2H, CHH–S–CHH), 4.79 (d, J = 13.2 Hz, 3H, CHH–S–CHH), 4.76 (s, 1H, OH), 3.80 (s, 3H, OCH3), 2.21 (s, 6H, 2 × CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 158.5 (Ar–CqOMe), 151.3 (Ar–Cq), 137.1 (Ar–C), 136.0 (Ar–C), 127.6 (2 × Ar–C), 126.8(2 × Ar–C), 123.5 (2 × Ar–Cq), 114.1 (2 × Ar–CH), 76.7 (CH2–SO2–CH2), 55.3 (OCH3), 36.3 (Cq), 16.1 (2 × CH3); HRMS (APCI) m/z Calculated for C18H19SO4– [M – H]−: 331.1010, Found: 331.0995.
3-(4-Hydroxy-3-isopropylphenyl)-3-(4-methoxyphenyl)thietane 1,1-dioxide (3ad)
Performed using general procedure B with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and 2-isopropylphenol (0.081 mL, 0.60 mmol, 3 equiv). Purification by flash column chromatography (40% EtOAc/hexane) afforded diarylthietane dioxide 3ad as a white solid (68.8 mg, 99%). Rf = 0.15 (40% EtOAc/hexane); mp = 165–168 °C; IR (film)/cm–1 3397 (OH), 3024, 2962, 1607, 1510, 1463, 1422, 1311, 1241, 1183, 1132, 1024, 845, 818, 777, 647, 541, 484; 1H NMR (400 MHz, CDCl3) δ 7.17 (d, J = 8.8 Hz, 2H, Ar–H), 7.06 (d, J = 2.6 Hz, 1H, Ar–H), 6.93–6.83 (m, 3H, Ar–H), 6.68 (d, J = 8.3 Hz, 1H, Ar–H), 4.95 (s, 1H, OH), 4.89–4.77 (m, 4H, 2 × CHH–SO2–CHH), 3.80 (s, 3H, OCH3), 3.17 (p, J = 6.9 Hz, 1H, CH), 1.21 (d, J = 6.9 Hz, 6H, 2 × CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 158.5 (Ar–Cq), 152.0 (Ar–Cq), 137.0 (2 × Ar–Cq), 135.0 (Ar–Cq), 127.7 (2 × Ar–C), 124.9 (Ar–C), 124.8 (Ar–C), 115.4 (Ar–C), 114.2 (2 × Ar–C), 77.2 (2 × SO2–CH2), 55.3 (O–CH3), 36.4 (Cq), 27.4 (CH), 22.4 (2 × CH3); HRMS(APCI) m/z Calculated for C19H21O4S [M – H]+: 345.1155; Found: 345.1158.
3-(4-Hydroxy-5-isopropyl-2-methylphenyl)-3-(4-methoxyphenyl)thietane 1,1-dioxide (3ae)
Performed using general procedure B with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and thymol (90.0 mg, 0.60 mmol, 3 equiv). Purification by flash column chromatography (25–30% EtOAc/pentane) afforded diarylthietane dioxide 3ae as a yellow solid (58.4 mg, 81%). Rf = 0.43 (30% EtOAc/pentane); mp = 200–204 °C; IR (film)/cm–1 3357 (OH), 2958, 1610, 1582, 1510, 1461, 1408, 1303, 1223, 1183, 1156, 1129, 1107, 1016, 830, 807, 785, 551, 485; 1H NMR (400 MHz, CDCl3) δ 7.25 (d, J = 8.9 Hz, 2H, Ar–H), 7.12 (s, 1H, Ar–H), 6.85 (d, J = 8.9 Hz, 2H, Ar–H), 6.59 (s, 1H, Ar–H), 4.90 (d, J = 14.7 Hz, 2H, CHH–SO2–CHH), 4.72 (d, J = 14.7 Hz, 2H, CHH–SO2–CHH), 3.80 (s, 3H, OCH3), 3.26 (sept, J = 6.9 Hz, 1H, CH), 1.85 (s, 3H, CH3), 1.33 (d, J = 6.9 Hz, 6H, 2 × CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 158.5 (Ar–Cq), 152.3 (Ar–Cq), 136.1 (Ar–Cq), 134.7 (Ar–Cq), 133.9 (Ar–Cq), 131.5 (Ar–Cq), 127.3 (2 × Ar–C), 124.9 (Ar–C), 119.5 (2 × Ar–C), 114.0 (Ar–C), 76.3 (CH2–SO2), 55.3 (OCH3), 36.5 (Cq), 27.2 (CH), 22.6 (2 × CH3), 20.5 (CH3); HRMS(ES-ToF) m/z Calculated for C20H25O4S [M + H]+: 361.1474; Found: 361.1479.
3-(4-Hydroxy-2-isopropyl-5-methylphenyl)-3-(4-methoxyphenyl)thietane 1,1-dioxide (3af)
Performed using general procedure B with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and carvacrol (0.092 mL, 0.60 mmol, 3 equiv). Purification by flash column chromatography (25–30% EtOAc/pentane) afforded diarylthietane dioxide 3af as a white solid (39.6 mg, 55%). Rf = 0.43 (30% EtOAc/pentane); mp = 190–195 °C; IR (film)/cm–1 3491 (OH), 2958, 2867, 1610, 1572, 1511, 1304, 1280, 1188, 1136, 1099, 1036, 905, 826, 784, 532, 476; 1H NMR (400 MHz, CDCl3) δ 7.23 (d, J = 8.9 Hz, 2H, Ar–H), 7.05 (s, 1H, Ar–H), 6.81 (d, J = 8.9 Hz, 2H, Ar–H), 6.71 (s, 1H, Ar–H), 4.84 (d, J = 13.6 Hz, 2H, CHH–SO2–CHH), 4.70 (d, J = 14.5 Hz, 2H, CHH–SO2–CHH), 3.77 (s, 3H, OCH3), 2.30 (sept, J = 8.9 Hz, 1H, CH), 2.28 (s, 3H, CH3), 0.85 (d, J = 6.7 Hz, 6H, 2 × CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 158.4 (Ar–Cq), 153.8 (Ar–Cq), 146.6 (Ar–Cq), 137.1 (Ar–Cq), 132.8 (Ar–Cq), 129.0 (Ar–C), 127.3 (2 × Ar–C), 120.7 (Ar–Cq), 114.9 (Ar–C), 114.0 (2 × Ar–C), 76.6 (CH2–SO2), 55.3 (OCH3), 36.2 (Cq), 30.0 (CH), 23.8 (2 × CH3), 15.6 (CH3); HRMS(APCI) m/z Calculated for C20H25O4S [M + H]+: 360.1468; Found: 360.1461.
3-(2-Hydroxy-5-methylphenyl)-3-(4-methoxyphenyl)thietane 1,1-dioxide (3ag)
Performed using general procedure B with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and p-cresol (0.062 mL, 0.60 mmol, 3 equiv). Purification by flash column chromatography (2–5% Et2O/CH2Cl2) afforded diarylthietane dioxide 3ag a white solid (54.4 mg, 90%). Rf = 0.56 (10% Et2O/CH2Cl2); mp = 178–180 °C; IR (film)/cm–1 3376, 3040, 2922, 1608, 1509, 1414, 1388, 1299, 1250, 1220, 1183, 1120, 1029, 841, 814, 768, 632, 614, 546; 1H NMR (400 MHz, DMSO-d6) δ 9.51 (s, 1H, OH), 7.39 (d, J = 8.9 Hz, 2H, 2 × Ar–H), 7.15 (d, J = 1.8 Hz, 1H, Ar–H), 6.90 (dd, J = 8.5, 1.8 Hz, 1H, Ar–H), 6.85 (d, J = 8.9 Hz, 2H, 2 × Ar–H), 6.66 (d, J = 8.5 Hz, 1H, Ar–H), 4.84 (s, 4H, CHH–S–CHH), 3.71 (s, 3H, OCH3), 2.23 (s, 3H, CH3); 13C{1H} (101 MHz, DMSO) δ 158.1 (Ar–Cq), 152.5 (Ar–Cq), 136.5 (Ar–Cq), 130.9 (Ar–C), 129.3 (Ar–C), 128.3 (Ar–C), 128.2 (2 × Ar–C), 128.0 (Ar–Cq), 116.4 (Ar–C), 113.9 (2 × Ar–C), 75.0 (CHH–S–CHH), 55.5 (OCH3), 36.1 (Cq), 20.7 (CH3); HRMS (ESI) m/z Calculated for C17H17O4S [M – H]−: 317.0853; Found: 317.0851.
3-(2-Hydroxy-5-methoxyphenyl)-3-(4-methoxyphenyl)thietane 1,1-dioxide (3ah)
Performed using general procedure B with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and 4-methoxyphenol (74.5 mg, 0.60 mmol, 3 equiv). Purification by flash column chromatography (2–5% Et2O/CH2Cl2) afforded diarylthietane dioxide 3ah as a white solid (53.7 mg, 80%). Rf = 0.56 (10% Et2O/CH2Cl2); mp = 178–180 °C; IR (film)/cm–1 3410 (OH), 2956, 2928, 1608, 1511, 1424, 1308, 1253, 1209, 1186, 1167, 1134, 1033, 811, 545, 501; 1H NMR (400 MHz, acetone-d6) δ 7.49 (d, J = 9.1 Hz, 2H, 2 × Ar–H), 6.99 (d, J = 2.9 Hz, 1H Ar–H), 6.85 (d, J = 9.1 Hz, 2H, 2 × Ar–H), 6.81–6.71 (m, 2H, 2 × Ar–H), 4.95 (d, J = 14.9 Hz, 2H, CHH–S–CHH), 4.83 (d, J = 14.9 Hz, 2H, CHH–S–CHH), 3.77 (s, 3H, OCH3), 3.76 (s, 3H, OCH3); 13C{1H} NMR (101 MHz, acetone-d6) δ 158.4 (Ar–Cq), 153.1 (Ar–Cq), 148.1 (Ar–Cq), 136.2 (Ar–Cq), 131.8 (Ar–Cq), 127.8 (2 × Ar–C), 116.8 (Ar–C), 113.8 (Ar–C), 113.4 (2 × Ar–C), 113.3 (Ar–C), 74.8 (2 × C–SO2), 55.1 (OCH3), 54.6 (OCH3), 35.7 (Cq); HRMS (ESI) m/z Calculated for C17H17O5S [M – H]−: 333.0802; Found: 333.0802.
(8R,9S,13S,14S)-3-Hydroxy-2-(3-(4-methoxyphenyl)-1,1-dioxidothietan-3-yl)-13-methyl-6,7,8,9,11,12,13,14,15,16-decahydro-17H-cyclopenta[a]phenanthren-17-one (3ai)
Performed using general procedure B with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and estrone (162 mg, 0.60 mmol, 3 equiv). Purification by flash column chromatography (50% acetone/pentane) afforded diarylthietane dioxide 3ai as a white solid (30.9 mg, 57%). Rf = 0.23 (50% acetone/pentane); IR (film)/cm–1 3402 (OH), 2929, 2861, 1734, 1610, 1511, 1416, 1315, 1253, 1217, 1186, 1143, 1122, 1033, 828, 734, 545; 1H NMR (400 MHz, CDCl3) δ 7.31 (d, J = 8.5 Hz, 2H, Ar–H), 7.08 (s, 1H, Ar–H), 6.83 (d, J = 8.5 Hz, 2H, Ar–H), 6.48 (s, 1H, Ar–H), 5.18 (s, 1H, OH), 4.97–4.84 (m, 2H, CHH–SO2–CHH), 4.75 (ddd, J = 14.3, 10.1, 3.7 Hz, 2H, CHH–SO2–CHH), 3.76 (s, 3H, OCH3), 2.90–1.34 (m, 15H), 0.92 (s, 3H, CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 221.0 (Cq=O), 158.5 (Ar–Cq), 150.7 (Ar–Cq), 137.9 (Ar–Cq), 135.7 (Ar–Cq), 132.4 (Ar–Cq), 127.6 (Ar–Cq), 127.4 (2 × Ar–C), 124.5 (Ar–C), 116.9 (Ar–C), 114.0 (2 × Ar–C), 75.6 (2 × C–SO2), 55.3 (OCH3), 50.3 (CH), 48.0 (Cq), 44.0 (CH), 38.3 (CH), 35.9 (CH2), 35.4 (CH2), 31.5 (CH2), 29.0 (CH2), 26.3 (Cq), 26.1 (CH2), 21.6 (CH2), 13.9 (CH3). HRMS (APCI) m/z Calculated for C28H32SO5 [M + H]+: 481.2043; Found: 481.2041.
3-(3,4-Dihydroxyphenyl)-3-(4-methoxyphenyl)thietane 1,1-dioxide (3aj)
Performed using general procedure B with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and catechol (66.0 mg, 0.60 mmol, 3 equiv). Purification by flash column chromatography (1–10% Et2O/CH2Cl2) afforded diarylthietane dioxide 3aj a white solid (47.4 mg, 78%). Rf = 0.29 (5% Et2O/CH2Cl2); mp = 182–186 °C; IR (film)/cm–1 3396 (OH), 2959, 2838, 1689, 1607, 1512, 1435, 1293, 1251, 1220, 1183, 1125, 1030, 828, 812, 785, 634, 546, 486; 1H NMR (400 MHz, MeOD) δ 7.26 (d, J = 8.9 Hz, 2H, Ar–H), 6.88 (d, J = 8.7 Hz, 2H, Ar–H), 6.77–6.62 (m, 3H, Ar–H), 4.80 (s, 4H, 2 × CHH–SO2–CHH), 3.76 (s, 3H, O–CH3); 13C{1H} NMR (101 MHz, MeOD) δ 159.9 (Ar–Cq), 146.5 (Ar–Cq), 145.4 (Ar–Cq), 138.8 (Ar–Cq), 138.4 (Ar–Cq), 128.9 (2 × Ar–C), 118.9 (Ar–C), 116.2 (Ar–C), 115.2 (Ar–C), 115.0 (2 × Ar–C), 77.2 (2 × SO2–CH2), 55.7 (Cq), 37.7 (O–CH3); HRMS (APCI) m/z Calculated for C16H15O5S [M – H]+: 319.0646; Found: 319.0640.
3-(2,4-Dihydroxyphenyl)-3-(4-methoxyphenyl)thietane 1,1-dioxide (3ak)
Performed using general procedure B with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and resorcinol (66.0 mg, 0.60 mmol, 3 equiv). The reaction was quenched by the addition of sat. aq. NaHCO3 (15 mL), then extracted with EtOAc (3 × 15 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated in vacuo using a rotary evaporator. Purification by flash column chromatography (1–10% Et2O/CH2Cl2) afforded diarylthietane dioxide 3ak white solid (43.2 mg, 71%). Rf = 0.29 (5% Et2O/CH2Cl2); IR (film)/cm–1 3401 (OH), 2963, 1607, 1513, 1303, 1251, 1217, 1186, 1130, 1114, 1031, 832, 544; 1H NMR (400 MHz, acetone-d6) δ 7.41 (d, J = 8.9 Hz, 2H, 2 × Ar–H), 7.18 (d, J = 8.7 Hz, 1H, Ar–H), 6.83 (d, J = 8.9 Hz, 2H, 2 × Ar–H), 6.40 (d, J = 3.3 Hz, 2H, 2 × Ar–H), 4.86 (d, J = 14.6 Hz, 2H, CHH–S–CHH), 4.76 (d, J = 14.4 Hz, 2H, CHH–S–CHH), 3.73 (s, 3H, OCH3); 13C{1H} NMR (101 MHz, acetone-d6) δ 158.2 (Ar–Cq), 158.0 (Ar–Cq), 155.4 (Ar–Cq), 137.1 (Ar–Cq), 128.3 (Ar–C), 127.7 (2 × Ar–C), 122.4 (Ar–Cq), 113.4 (2 × Ar–C), 106.6 (Ar–C), 103.5 (Ar–C), 75.2 (2 × S–CH2), 54.6 (OCH3), 34.9 (Cq); HRMS(ES-ToF) m/z Calculated for C16H17O5S [M + H]+: 321.0797; Found: 321.0790.
3-(2,4-Dimethoxyphenyl)-3-(4-methoxyphenyl)thietane 1,1-dioxide (3al)
Performed using general procedure B with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and 1,3-dimethoxybenzene (84.7 mg, 0.60 mmol, 3.0 equiv), with the reaction conducted at 40 °C. Purification by flash column chromatography (25–30% EtOAc/hexane) afforded diarylthietane dioxide 3al as a pale pink oil (68.4 mg, 98%). Rf = 0.20 (35% EtOAc/hexane); IR (film)/cm–1 3001, 2960, 2837, 1608, 1582, 1508, 1461, 1416, 1393, 1311 (SO2), 1252, 1208, 1185, 1156, 1126, 1030, 970, 912, 830, 804, 731, 641, 543, 503; 1H NMR (400 MHz, CDCl3) δ 7.26–7.24 (2 H, d, J = 8.8 Hz, 2 × Ar–C), 7.11–7.09 (1 H, d, J = 8.5 Hz, Ar–H), 6.83–6.81 (2 H, d, J = 8.8 Hz, 2 × Ar–H), 6.53–6.50 (1 H, dd, J = 8.5, 2.3 Hz, Ar–H), 6.46 (1 H, d, J = 2.3, Ar–H), 4.86–4.82 (2 H, d, J = 14.6 Hz, CHH–SO2–CHH), 4.74–4.70 (2 H, d, J = 14.6 Hz, CHH–SO2–CHH), 3.82 (3 H, s, OCH3), 3.78 (3 H, s, OCH3), 3.70 (3 H, s, OCH3); 13C{1H} NMR (101 MHz, CDCl3) δ 160.7 (Ar–CqOMe), 158.2 (Ar–CqOMe), 157.7 (Ar–CqOMe), 136.2 (Ar–Cq), 127.8 (Ar–C), 127.3 (2 × Ar–C), 124.3 (Ar–Cq), 113.8 (2 × Ar–C), 104.0 (Ar–C), 99.8 (Ar–C), 75.7 (CH2–SO2–CH2), 55.4 (OCH3), 55.3 (OCH3), 55.2 (OCH3), 35.0 (Cq); HRMS (ESI) m/z Calculated for C18H21O5S [M + H]: 349.1110, Found: 349.1110.
3-(4-Methoxyphenyl)-3-(2,4,6-trimethoxyphenyl)thietane 1,1-dioxide (3am)
Performed using general procedure B with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and 1,3,5-trimethoxybenzene (101 mg, 0.60 mmol, 3 equiv), with the reaction conduct at 40 °C. Purification by flash column chromatography (1–10% Et2O/CH2Cl2) afforded diarylthietane dioxide 3am as a white solid (68.8 mg, 91%). Rf = 0.29 (5% Et2O/CH2Cl2); mp = 198–206 °C; IR (film)/cm–1 2942, 2836, 1608, 1585, 1459, 1414, 1296, 1234, 1117, 1035, 968, 817, 633, 549, 522; 1H NMR (400 MHz, CDCl3) δ 7.48 (d, J = 8.6 Hz, 2H, Ar–H), 6.83 (d, J = 8.6 Hz, 2H, Ar–H), 6.15 (s, 2H, 2 × Ar–H), 4.93–4.70 (m, 4H, 2 × CHH–SO2–CHH), 3.81 (d, J = 3.5 Hz, 9H, 3 × O–CH3), 3.78 (s, 3H, O–CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 160.6 (Ar–Cq), 158.3 (Ar–Cq), 158.0 (2 × Ar–Cq), 136.1 (Ar–Cq), 127.1 (2 × Ar–C), 113.7 (2 × Ar–C), 113.6 (Ar–Cq), 91.5 (2 × Ar–C), 76.0 (2 × SO2–CH2), 55.7 (2 × O–CH3), 55.4 (O–CH3), 55.2 (O–CH3), 34.2 (Cq); HRMS(ESI) m/z Calculated for C19H23O6S [M + H]+: 379.1215; Found: 379.1201.
3-(4-Methoxyphenyl)-3-(1-methyl-1H-indol-3-yl)thietane 1,1-dioxide (3an)
Performed using general procedure B with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and N-methyl indole (0.075 mL, 0.60 mmol, 3 equiv). Purification by flash column chromatography (2–5% Et2O/CH2Cl2) afforded diarylthietane dioxide 3an as a white solid (37.5 mg, 55%). Rf = 0.56 (10% Et2O/CH2Cl2); mp = 185–188 °C; IR (film)/cm–1 3021, 1609, 1514, 1316, 1256, 1219, 1127, 1101, 1022, 825, 749, 642, 549; 1H NMR (400 MHz, CDCl3) δ 7.37–7.31 (m, 3H, Ar–H), 7.28–7.24 (m, 1H, Ar–H), 7.22 (d, J = 8.1 Hz, 1H, Ar–H), 7.05 (ddd, J = 8.0, 6.9, 1.0 Hz, 1H, Ar–H), 6.92 (s, 1H, Ar–H), 6.86 (d, J = 8.9 Hz, 2H, Ar–H), 4.95–4.79 (m, 4H, 2 × CHH–SO2–CHH), 3.79 (s, 6H, O–CH3 and N–CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 158.6 (Ar–Cq), 138.1 (Ar–Cq), 135.9 (Ar–Cq), 127.7 (2 × Ar–C), 127.3 (Ar–C), 125.3 (Ar–Cq), 122.3 (Ar–C), 119.7 (Ar–C), 119.7 (Ar–C), 117.8 (Ar–Cq), 114.0 (2 × Ar–C), 109.9 (Ar–C), 77.0 (2 × SO2–CH2), 55.3 (O–CH3), 32.9 (N–CH3), 31.8 (Cq); HRMS (ESI) m/z Calculated for C19H20O3SN [M + H]+: 342.1164; Found: 342.1164.
3-(4-Methoxyphenyl)-3-(5-methylfuran-2-yl)thietane 1,1-dioxide (3ao)
Performed using general procedure B with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and 2-methylfuran (54.1 μL, 0.60 mmol, 3 equiv). Purification by flash column chromatography (10% acetone/pentane) afforded diarylthietane dioxide 3ao as a brown oil (35.1 mg, 60%); Rf = 0.26 (20% acetone/pentane); IR (film)/cm–1 2959, 2837, 1609, 1512, 1321, 1251, 1211, 1133, 1027, 912, 831, 781, 731, 542, 493; 1H NMR (400 MHz, CDCl3) δ 7.20 (d, J = 8.8 Hz, 2H, Ar–H), 6.90 (d, J = 8.8 Hz, 2H, Ar–H), 5.93 (d, J = 3.1 Hz, 1H, CHfuran), 5.89 (d, J = 1.6 Hz, 1H, CHfuran), 4.81 (d, J = 14.1 Hz, 2H, CHH–SO2–CHH), 4.74–4.61 (m, 2H, CHH–SO2–CHH), 3.81 (s, 3H, OCH3), 2.26 (s, 3H, CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 158.9 (Ar–Cq), 153.2 (Ar–Cq), 153.0 (Ar–Cq), 133.6 (Ar–Cq), 127.6 (2 × Ar–C), 114.2 (2 × Ar–C), 108.8 (Cfuran), 106.5 (Cfuran), 75.3 (CH2–SO2–CH2), 55.3 (OCH3), 33.0 (Cq), 13.6 (CH3); HRMS (APCI) m/z Calculated for C15H17SO4+ [M + H]+: 293.0842, Found: 293.0829.
3-(4-Methoxyphenyl)-3-(5-methylthiophen-2-yl)thietane 1,1-dioxide (3ap)
Performed using general procedure B with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and 2-methylthiophene (53.3 μL, 0.60 mmol, 3 equiv). Purification by flash column chromatography (10% acetone/pentane) afforded diarylthietane dioxide 3ap as a brown oil (30.9 mg, 50%). Rf = 0.26 (20% acetone/pentane); IR (film)/cm–1 3023, 2958, 2837, 1609, 1512, 1321, 1252, 1222, 1184, 1130, 1031, 831, 802, 557, 536, 484; 1H NMR (400 MHz, CDCl3) δ 7.21 (d, J = 8.8 Hz, 2H, Ar–H), 6.90 (d, J = 8.8 Hz, 2H, Ar–H), 6.80 (d, J = 3.5 Hz, 1H, CHthiophene), 6.57 (d, J = 3.0 Hz, 1H, CHthiophene), 4.85 (d, J = 13.8 Hz, 2H, CHH–SO2–CHH), 4.78 (d, J = 13.9 Hz, 2H, CHH–SO2–CHH), 3.81 (s, 3H, OCH3), 2.39 (d, J = 1.1 Hz, 3H, CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 158.9 (Ar–Cq), 147.6 (Ar–Cq), 140.6 (Ar–Cq), 136.1 (Ar–Cq), 127.5 (2 × Ar–C), 125.0 (Cthiophene), 124.9 (Cthiophene), 114.2 (2 × Ar–C), 77.7 (CH2–SO2–CH2), 55.3 (OCH3), 34.5 (Cq), 15.3 (CH3); HRMS (APCI) m/z Calculated for C15H16S2O3 [M + H]+: 309.0614; Found: 309.0611.
3-(4-Hydroxy-3-methylphenyl)-3-(2-methoxyphenyl)thietane 1,1-dioxide (3ba)
Performed using general procedure B with thietanol dioxide 2b (45.7 mg, 0.20 mmol, 1 equiv) and o-cresol (62.0 μL, 0.60 mmol, 3 equiv). Purification by flash column chromatography (10% EtOAc/pentane) afforded diarylthietane dioxide 3ba as a white solid (62.2 mg, 66%). Rf = 0.10 (10% EtOAc/pentane); mp = 192–195 °C; IR (film)/cm–1 3391 (OH), 3043, 2946, 1600, 1510, 1488, 1451, 1429, 1289, 1232, 1195, 1168, 1128, 1105, 1054, 1020, 972, 809, 759, 614, 496; 1H NMR (400 MHz, CDCl3) δ 7.30 (ddd, J = 8.2, 7.7, 1.7 Hz, 1H, Ar–H), 7.17 (dd, J = 7.7, 1.7 Hz, 1H, Ar–H), 7.07 (d, J = 2.6 Hz, 1H, Ar–H), 7.05–6.96 (m, 2H, 2 × Ar–H), 6.88 (dd, J = 8.2, 1.1 Hz, 1H, Ar–H), 6.62 (d, J = 8.4 Hz, 1H, Ar–H), 5.00 (s, 1H, OH), 4.86 (d, J = 14.8 Hz, 2H, CHH–S–CHH), 4.76 (d, J = 14.8 Hz, 2H, CHH–S–CHH), 3.74 (s, 3H, OCH3), 2.17 (s, 3H, CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 156.6 (Ar–Cq), 152.8 (Ar–Cq), 135.3 (Ar–Cq), 132.0 (Ar–Cq), 129.3 (Ar–C), 129.0 (Ar–C), 127.3 (Ar–C), 125.0 (Ar–C), 123.9 (Ar–Cq), 120.7 (Ar–C), 114.7 (Ar–C), 111.9 (Ar–C), 75.3 (CHH–S–CHH), 55.2 (OCH3), 35.6 (Cq), 16.1 (CH3); HRMS (ESI) m/z Calculated for C17H19O4S [M + H]+: 319.1009; Found: 319.1028.
3-(4-Hydroxy-3-methylphenyl)-3-(3-methoxyphenyl)thietane 1,1-dioxide (3ca)
Performed using general procedure B with thietanol dioxide 2c (45.7 mg, 0.20 mmol, 1 equiv) and o-cresol (62.0 μL, 0.60 mmol, 3 equiv). Purification by flash column chromatography (20% EtOAc/pentane) afforded diarylthietane dioxide 3ca as a white solid (34.9 mg, 55%). Rf = 0.15 (30% EtOAc/pentane); mp = 169–172 °C; IR (film)/cm–1 3444 (OH), 3024, 2958, 2838, 1664, 1585, 1510, 1489, 1431l, 1316, 1271, 1221, 1126, 1050, 814, 780, 731, 481, 445; 1H NMR (400 MHz, CDCl3) δ 7.29 (d, J = 7.9 Hz, 1H, Ar–H), 7.00 (d, J = 2.7 Hz, 1H, Ar–H), 6.95 (dd, J = 8.4, 2.7 Hz, 1H, Ar–H), 6.86 (ddd, J = 7.9, 2.0, 0.9 Hz, 1H, Ar–H), 6.82–6.77 (m, 1H, Ar–H), 6.76 (dd, J = 2.2, 0.9 Hz, 1H, Ar–H), 6.71 (d, J = 8.4 Hz, 1H, Ar–H), 4.96 (s, 1H, OH), 4.83 (s, 4H, CHH–S–CHH), 3.78 (s, 3H, OCH3), 2.21 (s, 3H, CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 159.9 (Ar–Cq), 153.1 (Ar–Cq), 146.6 (Ar–Cq), 136.2 (Ar–Cq), 130.1 (Ar–C), 129.3 (Ar–C), 125.3 (Ar–C), 124.5 (Ar–Cq), 118.7 (Ar–C), 115.1 (Ar–C), 113.4 (Ar–C), 111.8 (Ar–C), 76.6 (CHH–S–CHH), 55.3 (OCH3), 37.0 (Cq), 16.1 (CH3); HRMS (ESI) m/z Calculated for C17H19O4S [M + H]+: 319.1004; Found: 319.1009.
3-(Benzo[d][1,3]dioxol-5-yl)-3-(4-hydroxy-3-methylphenyl)thietane 1,1-dioxide (3da)
Performed using general procedure B with thietanol dioxide 2d (48.4 mg, 0.20 mmol, 1 equiv) and o-cresol (62.0 μL, 0.60 mmol, 3 equiv). Purification by flash column chromatography (1:1:5 Et2O/CH2Cl2/pentane) afforded diarylthietane dioxide 3da as colorless oil (55.2 mg, 83%). Rf = 0.19 (1:1:5 Et2O/CH2Cl2/pentane); IR (film)/cm–1 3431 (OH), 3023, 1609, 1503, 1484, 1436, 1313, 1238, 1212, 1149, 1112, 1034, 930, 906, 809, 769, 727, 596, 473, 439; 1H NMR (400 MHz, CDCl3) δ 7.08–6.88 (m, 2H, Ar–H), 6.83–6.63 (m, 4H, Ar–H), 5.95 (s, 2H, O–CH2–O), 4.97 (s, 1H, OH), 4.87–4.70 (m, 4H, CHH–SO2–CHH), 2.22 (s, 3H, CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 153.0 (Ar–Cq), 148.3 (Ar–Cq), 146.7 (Ar–Cq), 138.8 (Ar–Cq), 136.5 (Ar–Cq), 129.2 (Ar–C), 125.2 (Ar–C), 124.5 (Ar–Cq), 119.6 (Ar–C), 115.1 (Ar–C), 108.2 (Ar–C), 107.4 (Ar–C), 101.4 (O–CH2–O), 76.6 (CHH–SO2–CHH), 36.9 (Cq), 16.0 (CH3); HRMS (APCI) m/z Calculated for C17H15O5S [M – H]−:331.0646; Found: 331.0641.
3-(4-Hydroxy-3-methylphenyl)-3-(4-((triisopropylsilyl)oxy)phenyl)thietane 1,1-dioxide (3ea)
Performed using general procedure B with thietanol dioxide 2e (74.2 mg, 0.20 mmol, 1 equiv) and o-cresol (62.0 μL, 0.60 mmol, 3 equiv). Purification by flash column chromatography (20% EtOAc/pentane) afforded diarylthietane dioxide 3ea as a white solid (59.9 mg, 65%). Rf = 0.41 (30% EtOAc/pentane); mp = 124–127 °C; IR (film)/cm–1 3456, 2945, 2866, 1606, 1510, 1463, 1318, 1271, 1178, 1128, 914, 883, 837, 685; 1H NMR (400 MHz, CDCl3) δ 7.08 (d, J = 8.3 Hz, 2H, Ar–H), 6.95 (d, J = 6.8 Hz, 2H, Ar–H), 6.83 (d, J = 8.3 Hz, 2H, Ar–H), 6.70 (d, J = 8.4 Hz, 1H, Ar–H), 4.82 (s, 4H, CHH–SO2–CHH), 2.20 (s, 3H, CH3), 1.24 (hept, J = 7.3 Hz, 3H, CH), 1.09 (d, J = 7.3 Hz, 18H, CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 155.2 (Ar–Cq), 153.0 (Ar–Cq), 137.4 (Ar–Cq), 136.7 (Ar–Cq), 129.5 (Ar–C), 127.7 (2 × Ar–C), 125.4 (Ar–C), 124.4 (Ar–Cq), 120.1 (2 × Ar–C), 115.1 (Ar–C), 77.1 (CHH–SO2–CHH), 36.3 (Cq), 17.9 (6 × CH3), 16.0 (CH3), 12.6 (3 × CH); HRMS (APCI) m/z Calculated for C25H37O4SSi [M + H]+:461.2176; Found: 461.2171.
3-(4-Hydroxy-3-methylphenyl)-3-(p-tolyl)thietane 1,1-dioxide (3fa)
Performed using general procedure B with thietanol dioxide 2f (42.4 mg, 0.20 mmol, 1 equiv) and o-cresol (62.0 μL, 0.60 mmol, 3 equiv). Purification by flash column chromatography (20% EtOAc/pentane) afforded diarylthietane dioxide 3fa as a white solid (55.6 mg, 92%). Rf = 0.24 (30% EtOAc/pentane); mp = 159–163 °C; IR (film)/cm–1 3412 (OH), 2961, 2920, 1609, 1510, 1312, 1272, 1220, 1123, 915, 818, 732, 594, 473; 1H NMR (400 MHz, CDCl3) δ 7.14 (m, 4H, 4 × Ar–H), 6.99 (d, J = 2.6 Hz, 1H, Ar–H), 6.93 (dd, J = 8.3, 2.7 Hz, 1H, Ar–H), 6.67 (d, J = 8.3 Hz, 1H, Ar–H), 5.21 (s, 1H, OH), 4.83 (m, 4H, CHH–S–CHH), 2.32 (s, 3H, CH3), 2.19 (s, 3H, CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 153.0 (Ar–Cq), 142.0 (Ar–Cq), 137.0 (Ar–Cq), 136.2 (Ar–Cq), 129.6 (2 × Ar–C), 129.2 (Ar–C), 126.2 (2 × Ar–C), 125.1 (Ar–C), 124.5 (Ar–Cq), 115.1 (Ar–C), 76.6 (2 × S–CH2), 36.7 (Cq), 20.9 (CH3), 16.0 (CH3); HRMS(ESI) m/z Calculated for C17H19O3S [M + H]+: 303.1055; Found: 303.1057.
3-(4-Hydroxy-3-methylphenyl)-3-phenylthietane 1,1-dioxide (3ga)
Performed using general procedure B with thietanol dioxide 2g (39.6 mg, 0.20 mmol, 1 equiv) and o-cresol (62.0 μL, 0.60 mmol, 3 equiv). Purification by flash column chromatography (20% EtOAc/pentane) afforded diarylthietane dioxide 3ga as a white solid (33.3 mg, 73%). Rf = 0.15 (30% EtOAc/pentane); mp = 171–173 °C; IR (film)/cm–1 3433 (OH), 3015, 2952, 1504, 1446, 1306, 1271, 1217, 1197, 1169, 1120, 1103, 703, 557, 531, 468, 447, 412; 1H NMR (400 MHz, acetone-d6) δ 8.3 (s, 1H, Ar–H), 7.50–7.45 (m, 2H, 2 × Ar–H), 7.41–7.32 (m, 2H, 2 × Ar–H), 7.29–7.21 (m, 2H, 2 × Ar–H), 7.12 (dd, J = 8.3, 2.7 Hz, 1H, Ar–H), 6.80 (d, J = 8.3 Hz, 1H, Ar–H), 4.92 (s, 4H, CHH–S–CHH), 2.19 (s, 3H, CH3); 13C{1H} NMR (101 MHz, acetone-d6) δ 154.2 (Ar–Cq), 146.3 (Ar–Cq), 136.3 (Ar–Cq), 129.1 (Ar–C), 128.6 (2 × Ar–C), 126.6 (Ar–C), 126.4 (2 × Ar–C), 125.0 Ar–C), 124.6 (Ar–Cq), 114.6 (Ar–C), 75.6 (2 × S–CH2), 37.2 (Cq), 15.5 (CH3); HRMS(ESI) m/z Calculated for C16H17O3S [M + H]+: 289.0898; Found: 289.0896.
3-(4-Chlorophenyl)-3-(4-hydroxy-3-methylphenyl)thietane 1,1-dioxide (3ha)
Performed using general procedure B with thietanol dioxide 2h (46.5 mg, 0.20 mmol, 1 equiv) and o-cresol (62.0 μL, 0.60 mmol, 3 equiv). Purification by flash column chromatography (20% EtOAc/pentane) afforded diarylthietane dioxide 3ha as a white solid (41.9 mg, 65%). Rf = 0.15 (30% EtOAc/pentane); mp = 159–163 °C; IR (film)/cm–1 3393 (OH), 1610, 1510, 1493, 1394, 1308, 1265, 1246, 1217, 1128, 1090, 1016, 829, 811, 783, 593, 412; 1H NMR (400 MHz, acetone-d6) δ 7.48 (d, J = 8.4 Hz, 2H, 2 × Ar–H), 7.36 (d, J = 8.4 Hz, 2H, 2 × Ar–H), 7.21 (d, J = 2.6 Hz, 1H, Ar–H), 7.10 (dd, J = 8.3, 2.6 Hz, 1H, Ar–H), 6.80 (d, J = 8.3 Hz, 1H, Ar–H), 4.90 (s, 4H, CHH–S–CHH), 2.17 (s, 3H, CH3); 13C{1H} NMR (101 MHz, acetone-d6) δ 154.4 (Ar–Cq), 145.2 (Ar–Cq), 135.7 (Ar–Cq), 132.1 (Ar–Cq), 129.1 (Ar–C), 128.6 (2 × Ar–C), 128.4 (2 × Ar–C), 125.0 (Ar–C), 124.7 (Ar–Cq), 114.7 (Ar–C), 75.5 (2 × S–CH2), 37.1 (CH3), 15.5 (Cq); HRMS(ESI) m/z Calculated for C16H1235ClO3S [M – H]−: 321.0358; Found: 321.0358.
3-(2,4-Dimethoxyphenyl)-3-(4-((triisopropylsilyl)oxy)phenyl)thietane 1,1-dioxide (3el)
Performed using general procedure B with thietanol dioxide 2e (74.2 mg, 0.20 mmol, 1 equiv) and 1,3-dimethoxy benzene (79.0 μL, 0.60 mmol, 3 equiv). Purification by flash column chromatography (10% EtOAc/pentane) afforded diarylthietane dioxide 3el as a white solid (56.9 mg, 58%). Rf = 0.10 (10% EtOAc/pentane); mp = 112–115 °C; IR (film)/cm–1 2942, 2865, 1607, 1581, 1507, 1462, 1317, 1209, 1128, 1233, 913, 834, 684; 1H NMR (400 MHz, CDCl3) δ 7.13 (d, J = 8.5 Hz, 2H, Ar–H), 7.09 (d, J = 8.5 Hz, 1H, Ar–H), 6.78 (d, J = 8.5 Hz, 2H, Ar–H), 6.51 (dd, J = 8.5, 2.5 Hz, 1H, Ar–H), 6.45 (d, J = 2.4 Hz, 1H, Ar–H), 4.82 (d, J = 14.6 Hz, 2H, CHH–SO2–CHH), 4.70 (d, J = 14.6 Hz, 2H, CHH–SO2–CHH), 3.81 (s, 3H, OCH3), 3.65 (s, 3H, OCH3), 1.22 (hept, J = 13.7, 6.6 Hz, 3H, CH), 1.07 (d, J = 7.3 Hz, 18H, CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 161.0 (Ar–Cq), 158.1 (Ar–Cq), 155.1 (Ar–Cq), 137.1 (Ar–Cq), 128.2 (Ar–C), 127.5 (2 × Ar–C), 124.6 (Ar–Cq), 120.0 (2 × Ar–C), 104.2 (Ar–C), 100.2 (Ar–C), 76.1 (CHH–SO2–CHH), 55.7 (OCH3), 55.5 (OCH3), 35.1 (Cq), 18.2 (6 × CH3), 12.9 (3 × CH); HRMS (APCI) m/z Calculated for C26H39O5SSi [M + H]+:491.2282; Found: 491.2281.
3-(4-Chlorophenyl)-3-(2,4-dimethoxyphenyl)thietane 1,1-dioxide (3hl)
Performed using general procedure B with thietanol dioxide 2h (46.5 mg, 0.20 mmol, 1 equiv) and 1,3-dimethoxybenzene (39.2 μL, 0.60 mmol, 3 equiv). Purification by flash column chromatography (20% EtOAc/pentane) afforded diarylthietane dioxide 3hl as a white solid (44.5 mg, 63%). Rf = 0.15 (30% EtOAc/pentane); mp = 147–149 °C; IR (film)/cm–1 2961, 2838, 1608, 1582, 1504, 1465, 1437, 1416, 1312, 1210, 1128, 1029, 971, 911, 826, 729, 527; 1H NMR (400 MHz, CDCl3) δ 7.26 (s, 4H, 4 × Ar–H), 7.13 (d, J = 8.4 Hz, 1H, Ar–H), 6.53 (d, J = 8.4 Hz, 1H, Ar–H), 6.45 (s, 1H, Ar–H), 4.82 (d, J = 16.6 Hz, 2H, 2 × Ar–H), 4.70 (d, J = 16.6 Hz, 2H, 2 × Ar–H), 3.82 (s, 3H, OCH3), 3.68 (s, 3H, OCH3); 13C{1H} NMR (101 MHz, CDCl3) δ 161.0 (Ar–Cq), 157.6 (Ar–Cq), 142.8 (Ar–Cq), 132.8 (Ar–Cq), 128.6 (2 × Ar–C), 127.7 (Ar–C), 127.6 (2 × Ar–C), 123.5 (Ar–Cq), 104.2 (Ar–C), 99.9 (Ar–C) 75.5 (2 × S–CH2), 55.5 (OCH3), 55.4 (OCH3), 35.3 (Cq); HRMS (ESI) m/z Calculated for C17H18O4S35Cl [M + H]+: 353.0614; Found: 353.0606.
3-(4-Chlorophenyl)-3-(2,4,6-trimethoxyphenyl)thietane 1,1-dioxide (3hm)
Performed using general procedure B with thietanol dioxide 2h (46.5 mg, 0.20 mmol, 1 equiv) and 1,3,5-trimethoxybenzene (101 mg, 0.60 mmol, 3 equiv). Purification by flash column chromatography (20% EtOAc/pentane) afforded diarylthietane dioxide 3hm as a white solid (60.8 mg, 79%). Rf = 0.15 (30% EtOAc/pentane); mp = 234–238 °C; IR (film)/cm–1 2971, 2939, 2842, 1608, 1588, 1459, 1299, 1120, 1060, 1034, 1012, 814, 524; 1H NMR (400 MHz, CDCl3) δ 7.47 (d, J = 8.5 Hz, 2H, 2 × Ar–H), 7.24 (d, J = 7.2 Hz, 2H, 2 × Ar–H), 6.12 (s, 2H, 2 × Ar–H), 4.82 (d, J = 16.3 Hz, 2H, CHH–S–CHH), 4.72 (d, J = 16.3 Hz, 2H, CHH–S–CHH), 3.80 (s, 3H, OCH3), 3.79 (s, 6H, 2 × OCH3); 13C{1H} NMR (101 MHz, CDCl3) δ 160.9 (Ar–Cq), 157.9 (2 × Ar–Cq), 142.5 (Ar–Cq), 132.8 (Ar–Cq), 128.5 (2 × Ar–C), 127.5 (2 × Ar–C), 112.7 (Ar–Cq), 91.4 (2 × Ar–C), 75.8 (2 × S–CH2), 55.7 (2 × OCH3), 55.4 (OCH3), 34.6 (Cq); HRMS (ESI) m/z Calculated for C18H20SO535Cl [M + H]+: 383.0714; Found: 383.0723.
3-(4-Methoxyphenyl)-2H-thiete 1,1-dioxide (4a)
Performed using general procedure B with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) without nucleophile. Purification by flash column chromatography (10% Et2O/pentane) afforded thiete dioxide 4a as a white solid (33.6 mg, 80%). Rf = 0.47 (10% Et2O/CH2Cl2); mp = 191–193 °C; IR (film)/cm–1 3102, 3078, 3018, 2998, 2968, 2945, 2838, 1606, 1564, 1508, 1427, 1277 (S=O), 1253, 1207, 1184, 1149, 1120, 1028, 927, 903, 841, 782, 673, 499, 479, 443; 1H NMR (400 MHz, CDCl3) δ 7.42–7.40 (2 H, d, J = 8.5 Hz, 2 × Ar–H), 6.98–6.96 (2 H, d, J = 8.5, 2 × Ar–H), 6.81 (1 H, s, C=CHSO2), 4.77 (2 H, s, CH2SO2), 3.88 (3 H, s, OCH3); 13C{1H} NMR (101 MHz, CDCl3) δ 162.7 (Ar–CqOMe), 146.7 (Cq), 133.9 (Cq=CHSO2), 129.4 (2 × Ar–C), 121.5 (Cq), 114.6 (2 × Ar–C), 69.8 (CH2–SO2), 55.5 (OCH3). The observed characterization data (IR, 1H and 13C NMR) were consistent with that previously reported.14
Thiol Alkylation with Thietan-3-ol Dioxide: General Procedure C
Calcium(II) bis(trifluoromethanesulfonimide) (6.0 mg, 0.01 mmol, 0.05 equiv) and tetrabutylammonium hexafluorophosphate (4.0 mg, 0.01 mmol, 0.05 equiv) were added sequentially to a solution of thietane-ol dioxide (0.20 mmol, 1 equiv) and thiol (0.60 mmol, 3 equiv) in toluene (0.4 mL, 0.5 M) in reaction vial. The reaction vial was sealed under argon, and the mixture was heated at 40 °C for 4.5 h then cooled to rt. Sat. aq. NaHCO3 (15 mL) was added followed by CH2Cl2 (15 mL). The phases were separated and the aqueous layer was extracted with CH2Cl2 (2 × 15 mL). The organic layers were combined, dried over Na2SO4, filtered and concentrated in vacuo. Purification by flash column chromatography afforded the thietane dioxide thioether.
3-(4-Methoxyphenyl)-3-(p-tolylthio)thietane 1,1-dioxide (5aa)
Performed using general procedure C with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and 4-methylbenzenethiol (41.4 mg, 0.60 mmol, 3 equiv). Purification by flash column chromatography (20% EtOAc/pentane) afforded 3-(4-methoxyphenyl)-3-(p-tolylthio)thietane 1,1-dioxide 5aa as a white solid (55.1 mg, 82%). Rf = 0.30 (20% EtOAc/pentane); mp = 123–127 °C; 1H NMR (400 MHz, CDCl3) δ 7.06 (d, J = 7.8 Hz, 2H, Ar–H), 6.98 (d, J = 8.1 Hz, 2H, Ar–H), 6.90 (d, J = 8.8 Hz, 2H, Ar–H), 6.81 (d, J = 8.8 Hz, 2H, Ar–H), 4.61 (d, J = 14.4 Hz, 2H, CHH–S–CHH), 4.52 (d, J = 14.3 Hz, 2H, CHH–S–CHH), 3.81 (s, 3H, OCH3), 2.33 (s, 3H, CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 159.0 (Ar–Cq), 140.5 (Ar–Cq), 136.6 (2 × Ar–C), 133.9 (Ar–Cq), 129.9 (2 × Ar–C), 128.0 (2 × Ar–C), 127.3 (Ar–Cq), 113.7 (2 × Ar–C), 75.5 (CH2–S–CH2), 55.3 (OCH3), 40.5 (Cq), 21.3 (CH3). HRMS (APCI) m/z calculated for C17H22O3NS2 [M + NH4]+: 352.1036; Found 352.1039.
3-((4-Bromophenyl)thio)-3-(4-methoxyphenyl)thietane 1,1-dioxide (5ab)
Performed using general procedure C with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and 4-methylbenzenethiol (41.4 mg, 0.60 mmol, 3 equiv). Purification by flash column chromatography (20% EtOAc/pentane) afforded 3-(4-methoxyphenyl)-3-(p-tolylthio)thietane 1,1-dioxide 5ab as a white solid (72.7 mg, 91%). Rf = 0.30 (20% EtOAc/pentane); mp = 123–127 °C; 1H NMR (400 MHz, CDCl3) δ 7.37 (d, J = 8.4 Hz, 2H, 2 × Ar–H), 6.90 (dd, J = 8.6, 7.7 Hz, 4H, 4 × Ar–H), 6.82 (d, J = 8.9 Hz, 2H, 2 × Ar–H), 4.63 (d, J = 14.7 Hz, 2H, CHH–S–CHH), 4.51 (d, J = 14.7 Hz, 2H, CHH–S–CHH), 3.82 (s, 3H, OCH3); 13C{1H} NMR (101 MHz, CDCl3) δ 159.1 (Ar–Cq), 137.8 (2 × Ar–C), 133.5 (Ar–Cq), 132.3 (2 × Ar–C), 129.8 (Ar–Cq), 128.0 (2 × Ar–C), 125.1 (Ar–Cq), 113.9 (2 × Ar–C), 75.7 (CHH–S–CHH), 55.4 (OCH3), 40.8 (Cq); HRMS (APCI) m/z calculated for C16H19O3N81BrS2 [M + NH4]+: 417.9964; Found 417.9963.
3-(Benzylthio)-3-(4-methoxyphenyl)thietane 1,1-dioxide (5ac)
Performed using general procedure C with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and phenylmethanethiol (35.2 μL, 0.60 mmol, 3 equiv). Purification by flash column chromatography (20% EtOAc/pentane) afforded 3-(benzylthio)-3-(4-methoxyphenyl)thietane 1,1-dioxide 5ac as a white solid (57.4 mg, 86%). Rf = 0.23 (20% EtOAc/pentane); mp = 121–124 °C; 1H NMR (400 MHz, CDCl3) δ 7.32–7.20 (m, 5H, Ar–H), 7.16 (d, J = 6.3 Hz, 2H, Ar–H), 6.95 (d, J = 8.8 Hz, 2H, Ar–H), 4.58 (d, J = 14.5 Hz, 2H, CHH–S–CHH), 4.33 (d, J = 14.6 Hz, 2H, CHH–S–CHH), 3.85 (s, 3H, O–CH3), 3.48 (s, 2H, S–CH2); 13C{1H} NMR (101 MHz, CDCl3) δ 159.1 (Ar–Cq), 135.9 (Ar–Cq), 132.5 (Ar–Cq), 129.0 (2 × Ar–C), 128.7 (2 × Ar–C), 128.1 (2 × Ar–C), 127.5 (Ar–C), 114.1 (2 × Ar–C), 76.7 (CH2–S–CH2), 55.4 (OCH3), 38.3 (Cq), 36.3 (S–CH2); HRMS (APCI) m/z calculated for C17H22O3NS2 [M + NH4]+: 352.1036; Found 352.1034.
Methyl 3-((3-(4-methoxyphenyl)-1,1-dioxidothietan-3-yl)thio)propanoate (5ad)
Performed using general procedure C with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and methyl 3-mercaptopropanoate (33.2 μL, 0.60 mmol, 3 equiv). Purification by flash column chromatography (20% EtOAc/pentane) afforded 3-(benzylthio)-3-(4-methoxyphenyl)thietane 1,1-dioxide 5ad as a light yellow gum (60.4 mg, 91%). Rf = 0.20 (30% EtOAc/pentane); 1H NMR (400 MHz, CDCl3) δ 7.22 (d, J = 8.8 Hz, 2H, Ar–H), 6.89 (d, J = 8.8 Hz, 2H, Ar–H), 4.73 (d, J = 14.6 Hz, 2H, CHH–S–CHH), 4.50 (d, J = 14.7 Hz, 2H, CHH–S–CHH), 3.80 (s, 3H, OCH3), 3.63 (s, 3H, OCH3), 2.53 (t, J = 7.3 Hz, 2H, CH2), 2.30 (t, J = 7.3 Hz, 2H, CH2); 13C{1H} NMR (101 MHz, CDCl3) δ 171.8 (C=O), 159.2 (Ar–Cq), 133.0 (Ar–Cq), 127.8 (2 × Ar–C), 114.3 (2 × Ar–C), 76.8 (2 × S–CH2), 55.4 (OCH3), 52.0 (OCH3), 38.1 (Cq), 32.9 (CH2), 26.6 (CH2); HRMS (APCI) m/z calculated for C14H22O5NS2 [M + NH4]+: 348.0934; Found 348.0937.
3-(((3s,5s,7s)-Adamantan-1-yl)thio)-3-(4-methoxyphenyl)thietane 1,1-dioxide (5ae)
Performed using general procedure C with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and 1-adamantanethiol (101 mg, 0.60 mmol, 3 equiv). Purification by flash column chromatography (20% EtOAc/pentane) afforded 3-(((3s,5s,7s)-adamantan-1-yl)thio)-3-(4-methoxyphenyl)thietane 1,1-dioxide 5ae as a white solid (54.5 mg, 72%). Rf = 0.20 (30% EtOAc/pentane); mp = 163–167 °C; IR (film)/cm–1 2903, 2848, 1720, 1608, 1511, 1451, 1325, 1254, 1213, 1182, 1100, 1031, 826, 731, 546, 498; 1H NMR (400 MHz, CDCl3) δ 7.40 (d, J = 8.8 Hz, 2H, 2 × Ar–H), 6.88 (d, J = 8.8 Hz, 2H, 2 × Ar–H), 4.75 (d, J = 14.5 Hz, 2H, CHH–S–CHH), 4.59 (d, J = 14.5 Hz, 2H, CHH–S–CHH), 3.82 (s, 3H, OCH3), 1.87 (s, 3H, 3 × CH), 1.63–1.44 (m, 12H, 6 × CH2); 13C{1H} NMR (101 MHz, CDCl3) δ 159.0 (Ar–Cq), 134.3 (Ar–Cq), 128.4 (2 × Ar–C), 113.8 (2 × Ar–C), 78.7 (CHH–S–CHH), 55.4 (OCH3), 50.4 (Cq), 43.2 (3 × CH2), 37.8 (Cq), 35.9 (3 × CH2), 29.5 (3 × CH); HRMS (TOF) m/z calculated for C20H30O3NS2 [M + NH4]+: 396.1667; Found 396.1679.
3-((4-Bromophenyl)thio)-3-(4-chlorophenyl)thietane 1,1-dioxide (5hb)
Performed using general procedure C with thietanol dioxide 2h (46.5 mg, 0.20 mmol, 1 equiv) and 4-bromothiophenol (56.7 mg, 0.60 mmol, 3 equiv), with the reaction conduct at 110 °C. Purification by flash column chromatography (20% EtOAc/pentane) afforded 3-((4-bromophenyl)thio)-3-(4-chlorophenyl)thietane 1,1-dioxide 5hb as a light yellow gum (52.5 mg, 65%). Rf = 0.20 (30% EtOAc/pentane); IR (film)/cm–1 3013, 2947, 1564, 1491, 1470, 1388, 1323, 1213, 1135, 1090, 1068, 1010, 908, 820, 770, 731, 490, 431; 1H NMR (400 MHz, CDCl3) δ 7.40 (d, J = 8.3 Hz, 2H, 2 × Ar–C), 7.29 (d, J = 7.2 Hz, 2H, 2 × Ar–C), 6.91 (t, J = 7.3 Hz, 4H, 4 × Ar–C), 4.62 (d, J = 13.0 Hz, 2H, CHH–S–CHH), 4.52 (d, J = 13.0 Hz, 2H, CHH–S–CHH); 13C{1H} NMR (101 MHz, CDCl3) δ 140.2 (Ar–Cq), 137.9 (2 × Ar–C), 137.8 (Ar–Cq), 134.2 (2 × Ar–C), 132.6 (2 × Ar–C), 128.9 (Ar–Cq), 128.1 (Ar–C), 125.6 (Ar–Cq), 75.5 (2 × S–CH2), 40.8 (Cq); HRMS (ESI) m/z calculated for C15H1279Br35ClO2S2 [M + Na]+: 424.9043; Found 424.9048.
Methyl 3-((3-(4-chlorophenyl)-1,1-dioxidothietan-3-yl)thio)propanoate (5hd)
Performed using general procedure C with thietanol dioxide 2h (46.5 mg, 0.20 mmol, 1 equiv) and methyl 3-mercaptopropanoate (33.2 μL, 0.60 mmol, 3 equiv), with the reaction conduct at 110 °C. Purification by flash column chromatography (20% EtOAc/pentane) afforded methyl 3-((3-(4-chlorophenyl)-1,1-dioxidothietan-3-yl)thio)propanoate 5hd as a light yellow gum (31.5 mg, 47%). Rf = 0.20 (30% EtOAc/pentane); IR (film)/cm–1 3019, 2952, 1731 (C=O), 1492, 1402, 1362, 1321, 1249, 1217, 1172, 1135, 1092, 1012, 829, 771, 531, 436; 1H NMR (400 MHz, CDCl3) δ 7.38 (d, J = 6.8 Hz, 2H, 2 × Ar–H), 7.27 (d, J = 7.7 Hz, 2H, 2 × Ar–H), 4.72 (d, J = 13.2 Hz, 2H, CHH–S–CHH), 4.53 (d, J = 13.2 Hz, 2H, CHH–S–CHH), 3.66 (s, 3H, OCH3), 2.55 (t, J = 7.3 Hz, 2H, CH2), 2.36 (t, J = 7.3 Hz, 2H, CH2); 13C{1H} NMR (101 MHz, CDCl3) δ 171.6 (C=O), 139.7 (Ar–Cq), 134.3 (Ar–Cq), 129.2 (2 × Ar–C), 127.9 (2 × Ar–C), 76.5 (2 × S–CH2), 38.1 (Cq), 32.7 (CH2), 26.5 (CH2); HRMS (ESI) m/z Calculated for C13H15O4S235Cl [M – H]−: 333.0028; Found: 333.0031.
Alcohol Alkylation with Thietan-3-ol Dioxide: General Procedure D
Alcohol (1.25 mmol, 5.0 equiv) in reaction vial was added to the solution of bis(trifluoromethanesulfonyl)amine (7.0 mg, 0.025 mmol, 0.1 equiv) in anhydrous acetonitrile (0.85 mL, 0.3 M) and then thietanol dioxide (0.20 mmol, 1 equiv) was added and the reaction vial was sealed, and the mixture stirred for 4.5 h at 50 °C then cooled to rt. Sat. aq. NaHCO3 (15 mL) was added followed by CH2Cl2 (15 mL). The phases were separated and the aqueous layer was extracted with CH2Cl2 (2 × 15 mL). The organic layers were combined, dried over Na2SO4, filtered and concentrated in vacuo. Purification by flash column chromatography afforded the thietane dioxide ether.
3-(4-Methoxyphenyl)-3-(3-phenylpropoxy)thietane 1,1-dioxide (6aa)
Performed using general procedure D with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and 3-phenyl-1-propanol (0.17 mL, 1.25 mmol, 5.0 equiv). Purification by flash column chromatography (10% EtOAc/pentane) afforded thietane dioxide ether 6aa (38.1 mg, 55%). Rf = 0.31 (15% EtOAc/pentane) as colorless oil; IR (film)/cm–1 3026, 2945, 1608, 1513, 1322, 1251, 1212, 1165, 1132, 1068, 1032, 834, 743, 701, 661, 561; 1H NMR (400 MHz, CDCl3) δ 7.33–7.24 (m, 4H, 4 × Ar–H), 7.24–7.19 (m, 1H, Ar–H), 7.17 (dd, J = 8.1, 1.4 Hz, 2H, 2 × Ar–H), 6.98–6.91 (m, 2H, 2 × Ar–H), 4.54–4.41 (m, 4H, CHH–S–CHH), 3.85 (s, 3H, OCH3), 3.07 (t, J = 6.1 Hz, 2H, OCH2), 2.70 (t, J = 7.5 Hz, 2H, CH2), 1.94–1.82 (m, 2H, CH2); 13C{1H} NMR (101 MHz, CDCl3) δ 159.8 (Ar–Cq), 141.3 (Ar–Cq), 130.5 (Ar–Cq), 128.5 (2 × Ar–C), 128.4 (2 × Ar–C), 127.6 (2 × Ar–C), 125.9 (Ar–C), 114.2 (2 × Ar–C), 73.8 (2 × S–CH2), 68.4 (Cq), 63.3 (OCH3), 55.4 (OCH2), 31.9 (CH2), 30.8 (CH2); HRMS (ESI) m/z Calculated for C19H26O4SN [M + NH4]+: 364.1583; Found: 364.1578.
3-Ethoxy-3-(4-methoxyphenyl)thietane 1,1-dioxide (6ab)
Performed using general procedure D with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and ethanol (0.073 mL, 1.25 mmol, 5.0 equiv). Purification by flash column chromatography (10% EtOAc/pentane) afforded thietane dioxide ether 6ab (21.0 mg, 41%). Rf = 0.31 (15% EtOAc/pentane) as colorless oil; IR (film)/cm–1 2974, 2932, 2838, 1610, 1515, 1312, 1251, 1213, 1181, 1135, 1031, 975, 833, 766, 662, 563, 486; 1H NMR (400 MHz, CDCl3) δ 7.29 (d, J = 8.4 Hz, 2H, 2 × Ar–H), 6.93 (d, J = 8.3 Hz, 2H, 2 × Ar–H), 4.47 (s, 4H, CHH–S–CHH), 3.83 (s, 3H, OCH3), 3.13 (q, J = 7.0 Hz, 2H, OCH2), 1.16 (t, J = 7.0 Hz, 3H, CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 159.9 (Ar–Cq), 130.8 (Ar–Cq), 127.5, (2 × Ar–C), 114.3 (2 × Ar–C), 74.1 (2 × S–CH2), 68.4 (Cq), 60.3 (OCH3), 55.4 (OCH2), 15.1 (CH3).
3-((4-Bromobenzyl)oxy)-3-(4-methoxyphenyl)thietane 1,1-dioxide (6ac)
Performed using general procedure D with thietanol dioxide 2a (45.7 mg, 0.20 mmol, 1 equiv) and 4-bromobenzyl alcohol (187 mg, 1.25 mmol, 5.0 equiv). Purification by flash column chromatography (10% EtOAc/pentane) afforded thietane dioxide ether 6ac (31.7 mg, 40%). Rf = 0.24 (15% EtOAc/pentane) as white solid; mp = 152–155 °C; IR (film)/cm–13025, 2957, 2838, 1608, 1515,1489,1321, 1250, 1211, 1133, 1033, 833, 807, 766, 513, 425; 1H NMR (400 MHz, CDCl3) δ 7.45 (d, J = 8.4 Hz, 2H, 2 × Ar–CH), 7.32 (d, J = 8.8 Hz, 2H, 2 × Ar–CH), 7.14 (d, J = 8.5 Hz, 2H, 2 × Ar–CH), 6.96 (d, J = 8.8 Hz, 2H, 2 × Ar–CH), 4.54 (s, 4H, CHH–SO2–CHH), 4.11 (s, 2H, OCH2), 3.85 (s, 3H, OCH3); 13C{1H} NMR (101 MHz, CDCl3) δ 160.1 (Ar–Cq), 135.8 (Ar–Cq), 131.6 (2 × Ar–C), 129.9 (Ar–Cq), 129.0 (2 × Ar–C), 121.8 (Ar–Cq), 114.5 (2 × Ar–C), 73.9 (2 × S–CH2), 69.2 (Cq), 66.1 (OCH2), 55.4 (OCH3); HRMS (ESI) m/z Calculated for C17H16O4SBr [M – H]−: 394.9958; Found: 394.9960.
Further Derivatization Reactions
3-(4-Methoxyphenyl)-3-(4-(pyridin-2-yloxy)phenyl)thietane 1,1-dioxide (7)
3-(4-Hydroxyphenyl)-3-(4-methoxyphenyl)thietane 1,1-dioxide 3ba (30.4 mg, 0.10 mmol, 1.0 equiv) and 2-iodopyridine (12.8 μL, 0.12 mmol, 1.2 equiv) were added to the solution of copper iodide (1.0 mg 0.005 mmol, 0.05 equiv), picolinic acid (1.2 mg, 0.01 mmol, 0.1 equiv), and potassium phosphate (42.4 mg, 0.2 mmol, 2.0 equiv) in 0.2 mL DMSO. The reaction was heated to 90 °C, leaving it stir for 24 h, before quenching the reaction with 10 mL water. The aqueous mixture was then extracted with EtOAc (3 × 10 mL). The combined organic layers were dried with Na2SO4, filtered and solvent removed under reduced pressure. Purification by flash chromatography (20% EtOAc/pentane) afforded 3-(4-methoxyphenyl)-3-(4-(pyridin-2-yloxy)phenyl)thietane 1,1-dioxide 7 (29.5 mg, 77%) as white solid. Rf = 0.22 (30% EtOAc/pentane); mp = 163–167 °C; IR (film)/cm–1 3016, 2954, 1605, 1504, 1459, 1428, 1316, 1273, 1210, 1183, 1129, 1027, 885, 836, 783, 546, 412; 1H NMR (400 MHz, CDCl3) δ 8.21 (d, J = 4.8 Hz, 1H, Arpyr–H), 7.73 (td, J = 8.0, 2.0 Hz, 1H, Arpyr–H), 7.30 (d, J = 8.8 Hz, 2H, 2 × Ar–H), 7.24 (d, J = 8.8 Hz, 2H, 2 × Ar–H), 7.14 (d, J = 8.7 Hz, 2H, 2 × Ar–H), 7.07–7.01 (m, 1H, Arpyr–H), 6.96 (d, J = 8.0 Hz, 1H, Arpyr–H), 6.92 (d, J = 8.8 Hz, 2H, 2 × Ar–H), 4.89 (s, 4H, CHH–S–CHH), 3.83 (s, 3H, OCH3); 13C{1H} NMR (101 MHz, CDCl3) δ 163.1 (Ar–Cq), 158.7 (Ar–Cq), 153.2 (Ar–Cq), 147.6 (Arpyr–C), 141.0 (Ar–Cq), 139.6 (Arpyr–C), 136.1 (Ar–Cq), 127.9 (4 × Ar–C), 121.3 (2 × Ar–C), 118.8 (Arpyr–C), 114.3 (2 × Ar–C), 111.9 (Arpyr–C), 77.0 (2 × S–CH2), 55.3 (OCH3), 36.5 (Cq); HRMS (ESI) m/z Calculated for C21H20O4SN [M + H]+: 382.1113; Found: 382.1105.
4-(3-(4-Methoxyphenyl)-1,1-dioxidothietan-3-yl)phenyl trifluoromethanesulfonate (8)
3-(4-Hydroxyphenyl)-3-(4-methoxyphenyl)thietane 1,1-dioxide 3ba (252 mg, 0.82 mmol, 1.0 equiv) and pyridine (0.133 mL, 1.64 mmol, 2 equiv) in CH2Cl2 (1.7 mL) was slowly added by the triflic anhydride (0.153 mL, 0.90 mmol, 1.1 equiv) at 0 °C. The reaction flask was warmed up to 25 °C using a water bath then left to stir for 3 h, before quenching the reaction with sat. NaHCO3. The aqueous mixture was extracted with Et2O (3 × 30 mL). The combined organic layers were dried with Na2SO4, filtered and solvent removed under reduced pressure. Purification by flash chromatography (10% EtOAc/pentane) afforded 4-(3-(4-methoxyphenyl)-1,1-dioxidothietan-3-yl)phenyl trifluoromethanesulfonate 8 (357 mg, 99%) as colorless liquid. Rf = 0.51 (20% EtOAc/pentane); IR (film)/cm–1 3015, 2969, 1608, 1515, 1502, 1414, 1321, 1265, 1208, 1129, 1032, 846, 827, 781, 762, 602, 544, 416; 1H NMR (400 MHz, CDCl3) δ 7.37 (d, J = 9.0 Hz, 2H, 2 × Ar–H), 7.25 (d, J = 9.0 Hz, 2H, 2 × Ar–H), 7.15 (d, J = 8.8 Hz, 2H, 2 × Ar–H), 6.92 (d, J = 8.8 Hz, 2H, 2 × Ar–H), 4.89 (d, J = 13.7 Hz, 2H, CHH–S–CHH), 4.80 (d, J = 13.7 Hz, 2H, CHH–S–CHH), 3.81 (s, 3H, OCH3); 13C{1H} NMR (101 MHz, CDCl3) δ 158.9 (Ar–Cq), 148.3 (Ar–Cq), 145.6 (Ar–Cq), 135.2 (Ar–Cq), 128.5 (2 × Ar–C), 127.8 (2 × Ar–C), 121.7 (2 × Ar–C), 114.5 (2 × Ar–C), 76.7 (2 × S–CH2), 55.3 (OCH3), 36.7 (Cq); 19F NMR (377 MHz, CDCl3) δ −72.8; HRMS (ESI) m/z Calculated for C17H14O6S2F3 [M – H]−: 435.0189; Found: 435.0186.
3-([1,1′-Biphenyl]-4-yl)-3-(4-methoxyphenyl)thietane 1,1-dioxide (9)
4-(3-(4-Methoxyphenyl)-1,1-dioxidothietan-3-yl)phenyl trifluoromethanesulfonate 8 (43.6 mg, 0.10 mmol, 1.0 equiv) and phenylboronic acid pinacol ester (30.6 mg, 0.15 mmol, 1.5 equiv) was added to a mixture of palladium(II) acetate (1.1 mg, 0.005 mmol, 0.05 equiv), SPhos (4.1 mg, 0.02 mmol, 0.1 equiv), and K3PO4 (84.9 mg, 0.20 mmol, 2.0 equiv) in dioxane/H2O (1.0 mL, 4:1). The reaction was heated to 65 °C and stirred for 24 h. The mixture was then filtered through Celite using Et2O (30 mL) and the solvent was removed under reduced pressure. Purification by flash chromatography (1:1:3 CH2Cl2/Et2O/pentane) afforded 3-([1,1′-biphenyl]-4-yl)-3-(4-methoxyphenyl)thietane 1,1-dioxide 9 (28.9 mg, 79%) as colorless oil; Rf = 0.55 (3:3:4 CH2Cl2/Et2O/pentane); IR (film)/cm–1 3029, 2960, 2837, 1608, 1511, 1487, 1396, 1321, 1252, 1223, 1183, 1135, 1031, 829, 736, 766, 699, 578, 498; 1H NMR (400 MHz, CDCl3) δ 7.57 (t, J = 7.5 Hz, 4H, 4 × Ar–H), 7.44 (t, J = 7.6 Hz, 2H, 2 × Ar–H), 7.39–7.31 (m, 3H, 3 × Ar–H), 7.29–7.19 (m, 2H, 2 × Ar–H), 6.90 (d, J = 6.8 Hz, 2H, 2 × Ar–H), 4.89 (s, 4H, 2 × CHH–S–CHH), 3.81 (s, 3H, OCH3); 13C{1H} NMR (101 MHz, CDCl3) δ 158.7 (Ar–Cq), 143.8 (Ar–Cq), 140.2 (Ar–Cq), 140.0 (Ar–Cq), 136.3 (Ar–Cq), 128.9 (2 × Ar–C), 127.6 (3 × Ar–C), 127.0 (2 × Ar–C), 126.9 (2 × Ar–C), 114.3 (2 × Ar–C), 76.7 (2 × S–CH2), 55.3 (OCH3), 36.8 (Cq); HRMS (ESI) m/z Calculated for C22H21O3S [M + H]+: 365.1211; Found: 365.1225.
3-(4-Methoxyphenyl)thietane-3-carboxylic acid 1,1-dioxide (10)
3-(4-Methoxyphenyl)-3-(5-methylfuran-2-yl)thietane 1,1-dioxide 3ao (180 mg, 0.60 mmol, 1.0 equiv) was added to the solution of sodium periodate (898 mg, 4.20 mmol, 7.0 equiv) in mixture solvent of 15 mL (1:1:2 heptane/EtOAc/water). Ruthenium chloride (6.2 mg, 0.03 mmol, 0.05 equiv) was added to the reaction tube at 0 °C. Then, the reaction tube was warmed up to 25 °C using a water bath and then left it stir for 16 h, before quenching the reaction with water. The aqueous mixture was added by 30 mL sat. NaS2O3, then extracted with EtOAc (3 × 10 mL). The combined organic layer was then extracted with 10 mL NaOH three times. The combined aqueous layers were acidified with 1 M HCl until the value of pH was lower than 7. The aqueous solution was then extracted with EtOAc (3 × 20 mL). The combined organic layers were dried with Na2SO4, filtered and solvent removed under reduced pressure to afford 3-(4-methoxyphenyl)thietane-3-carboxylic acid 1,1-dioxide 10 (86.1 mg, 56%) as a white solid. Rf = 0.18 (50% EtOAc/pentane); mp = 152–156 °C; IR (film)/cm–1 3193 (OH), 2960, 2915, 1735 (C=O), 1608, 1513, 1319, 1254, 1211, 1154, 1131, 1029, 833, 784, 734, 496, 442; 1H NMR (400 MHz, CDCl3) δ 7.23 (d, J = 8.9 Hz, 2H, 2 × Ar–H), 6.95 (d, J = 8.9 Hz, 2H, 2 × Ar–H), 4.97 (d, J = 14.6 Hz, 2H, CHH–S–CHH), 4.55 (d, J = 14.6 Hz, 2H, CHH–S–CHH), 3.84 (s, 3H, OCH3); 13C{1H} NMR (101 MHz, CDCl3) δ 159.9 (C=O), 129.1 (Ar–Cq), 127.9 (Ar–Cq), 114.7 (2 × Ar–C), 114.3 (2 × Ar–C), 72.9 (2 × S–CH2), 55.5 (OCH3), 39.6 (Cq); HRMS (ESI) m/z Calculated for C11H12O5S [M – H]−: 255.0333; Found: 255.0331.
Ethyl (3-(4-methoxyphenyl)-1,1-dioxidothietane-3-carbonyl)valinate (11)
3-(4-Methoxyphenyl)thietane-3-carboxylic acid 1,1-dioxide 10 (25.6 mg, 0.10 mmol, 1.0 equiv) and dl-valine ethyl ester hydrochloride (21.8 mg, 0.12 mmol, 1.2 equiv) were added to the solution of DIPEA (55.7 μL, 0.32 mmol, 3.2 equiv) and HATU (45.6 mg, 0.12 mmol, 1.2 equiv) in CH2Cl2 (0.1 mL) at 0 °C, leave it stir for 1 h. Then, the reaction flask was warmed up to 25 °C using a water bath and then left it stir for 23 h, before quenching the reaction with water. The aqueous mixture was extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were dried with Na2SO4, filtered and solvent removed under reduced pressure. Purification by flash chromatography (30–40% EtOAc/pentane) afforded ethyl (3-(4-methoxyphenyl)-1,1-dioxidothietane-3-carbonyl)valinate 11 (25.9 mg, 68%) as colorless oil. Rf = 0.31 (50% EtOAc/pentane); IR (film)/cm–1 3289 (NH), 2960, 1735 (C=O), 1640, 1608, 1541, 1508, 1465, 1444, 1396, 1318, 1252, 1219, 1191, 1131, 1031, 833, 800, 691, 669, 572, 449; 1H NMR (400 MHz, CDCl3) δ 7.28 (d, J = 8.8 Hz, 2H, 2 × Ar–H), 6.99 (d, J = 8.8 Hz, 2H, 2 × Ar–H), 5.76 (d, J = 8.6 Hz, 1H, S–CHH), 5.08 (d, J = 14.2 Hz, 1H, S–CHH), 4.92 (d, J = 14.2 Hz, 1H, S–CHH), 4.53 (dd, J = 13.9, 2.8 Hz, 1H, S–CHH), 4.47–4.40 (m, 2H, N–CH + NH), 4.20–4.06 (m, 2H, OCH2), 3.84 (s, 3H, OCH3), 2.08 (dqd, J = 13.8, 6.9, 4.8 Hz, 1H, CH), 1.24 (t, J = 7.1 Hz, 3H), 0.78 (d, J = 6.9 Hz, 3H, CH3), 0.66 (d, J = 6.9 Hz, 3H, CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 171.3 (C=O), 171.2 (C=O), 159.8 (Ar–Cq), 129.3 (Ar–Cq), 128.0 (2 × Ar–C), 115.1 (2 × Ar–C), 73.4 (S–CH2), 73.0 (S–CH2), 61.6 (OCH2), 57.8 (N–CH), 55.5 (OCH3), 39.4 (Cq), 31.1 (CH), 18.9 (CH3), 17.3 (CH3), 14.2 (CH3); HRMS (ESI) m/z Calculated for C18H26O6SN [M + H]+: 384.1481; Found: 384.1498.
Acknowledgments
We gratefullry acknowledge The Royal Society University Research Fellowship, URF\R\201019 (to J.A.B.), Pfizer, and Imperial College London for studentship funding (J.J.R.). P.S. is grateful for funding support provided by the Development and Promotion of Science and Technology Talents Project (DPST) and the Institute for the Promotion of Teaching Science and Technology (IPST).
Data Availability Statement
The data underlying this study are available in the published article, in its Supporting Information and openly available in the Imperial College London Research Data Repository at 10.14469/hpc/14599.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.4c01843.
X-ray Crystallography data; Copies of 1H and 13C NMR spectra (PDF)
Related Paper
A version of this manuscript was deposited on the preprint repository ChemRxiv.19
The authors declare no competing financial interest.
Supplementary Material
References
- Shearer J.; Castro J. L.; Lawson A. D. J.; MacCoss M.; Taylor R. D. Rings in Clinical Trials and Drugs: Present and Future. J. Med. Chem. 2022, 65, 8699–8712. 10.1021/acs.jmedchem.2c00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Rojas J. J.; Bull J. A. Oxetane in Drug Discovery Campaigns. J. Med. Chem. 2023, 66, 12697–12709. 10.1021/acs.jmedchem.3c01101. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wuitschik G.; Carreira E. M.; Wagner B.; Fischer H.; Parrilla I.; Schuler F.; Rogers-Evans M.; Müller K. Oxetane in Drug Discovery: Structural and Synthetic Insights. J. Med. Chem. 2010, 53, 3227–3246. 10.1021/jm9018788. [DOI] [PubMed] [Google Scholar]; c Carreira E. M.; Fessard T. C. Four-Membered Ring-Containing Spirocycles: Synthetic Strategies and Opportunities. Chem. Rev. 2014, 114, 8257–8322. 10.1021/cr500127b. [DOI] [PubMed] [Google Scholar]
- Francisco K. R.; Ballatore C. Thietanes and Derivatives thereof in Medicinal Chemistry. Curr. Top. Med. Chem. 2022, 22, 1219–1234. 10.2174/1568026622666220511154228. [DOI] [PubMed] [Google Scholar]
- Boezio A.; Taylor A. M.; Gunaydin H.; Zhang H.; Raynor K. D.; Shortsleeves K. C.; Dipietro L. V.; Pierce L. C. T.; Pabon N.; Mclean T. H.; Giordanetto F.; Pechersky Y.; Wang Q.; Laruvee A.; Chen F.; Maertens G.; Outin J.; Bertrand-Laperle M.; Pal M.; Chitale S.; Deninno M. P.. Pi3k-Alpha Inhibitors and Methods of Use Thereof. WO 2023039532 A1, March 16, 2023.
- Cohen F.; Aggen J. B.; Andrews L. D.; Assar Z.; Boggs J.; Choi T.; Dozzo P.; Easterday A. N.; Haglund C. M.; Hildebrandt D. J.; Holt M. C.; Joly K.; Jubb A.; Kamal Z.; Kane T. R.; Konradi A. W.; Krause K. M.; Linsell M. S.; Machajewski T. D.; Miroshnikova O.; Moser H. E.; Nieto V.; Phan T.; Plato C.; Serio A. W.; Seroogy J.; Shakhmin A.; Stein A. J.; Sun A. D.; Sviridov S.; Wang Z.; Wlasichuk K.; Yang W.; Zhou X.; Zhu H.; Cirz R. T. Optimization of LPXC Inhibitors for Antibacterial Activity and Cardiovascular Safety. ChemMedChem. 2019, 14, 1560–1572. 10.1002/cmdc.201900287. [DOI] [PubMed] [Google Scholar]
- Renold P.; Zambach W.; Maienfisch P.; Michel N.. Insecticidal Compound. WO 2009080250 A2, July 2, 2009.
- a Lassalas P.; Oukoloff K.; Makani V.; James M.; Tran V.; Yao Y.; Huang L.; Vijayendran K.; Monti L.; Trojanowski J. Q.; Lee V. M.; Kozlowski M. C.; Smith A. B.; Brunden K. R.; Ballatore C. Evaluation of Oxetan-3-ol, Thietan-3-ol, and Derivatives Thereof as Bioisosteres of the Carboxylic Acid Functional Group. ACS Med. Chem. Lett. 2017, 8, 864–868. 10.1021/acsmedchemlett.7b00212. [DOI] [PMC free article] [PubMed] [Google Scholar]; Also see:; b Francisco K. R.; Varricchio C.; Paniak T. J.; Kozlowski M. C.; Brancale A.; Ballatore C. Structure Preperty Relationships of N-Acylsulfonamides and Related Bioisosteres. Eur. J. Med. Chem. 2021, 218, 113399. 10.1016/j.ejmech.2021.113399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F.; Lin Z.; Wang F.; Zhao W.; Dong X. Four-membered Heterocycles-containing 4-Anilino-quinazoline Derivatives as Epidermal Growth Factor Receptor (EGFR) Kinase Inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 5385–5388. 10.1016/j.bmcl.2013.07.049. [DOI] [PubMed] [Google Scholar]
- a Croft R. A.; Mousseau J. J.; Choi C.; Bull J. A. Structurally Divergent Lithium Catalyzed Friedel-Crafts Reactions on Oxetan-3-ols: Synthesis of 3,3-Diaryloxetanes and 2,3-Dihydrobenzofurans. Chem.—Eur. J. 2016, 22, 16271–16276. 10.1002/chem.201604031. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Croft R. A.; Mousseau J. J.; Choi C.; Bull J. A. Oxetane Ethers are Formed Reversibly in the Lithium-Catalyzed Friedel-Crafts Alkylation of Phenols with Oxetanols: Synthesis of Dihydrobenzofurans, Diaryloxetanes, and Oxetane Ethers. Tetrahedron 2018, 74, 5427–5435. 10.1016/j.tet.2018.02.069. [DOI] [Google Scholar]; c Denis C.; Dubois M. A. J.; Voisin-Chiret A. S.; Bureau R.; Choi C.; Mousseau J. J.; Bull J. A. Synthesis of 3,3-Diarylazetidines by Calcium(II)-Catalyzed Friedel-Crafts Reaction of Azetidinols with Unexpected Cbz Enhanced Reactivity. Org. Lett. 2019, 21, 300–304. 10.1021/acs.orglett.8b03745. [DOI] [PubMed] [Google Scholar]; Also see:; d Liu S.; Zang Y.; Huang H.; Sun J. In(OTf)3-Catalyzed Synthesis of 2,3-Dihydro-1H-Benzo[e]Indoles and 2,3-Dihydrobenzofurans via [3 + 2] Annulation. Org. Lett. 2020, 22, 8219–8223. 10.1021/acs.orglett.0c02729. [DOI] [PubMed] [Google Scholar]
- a Croft R. A.; Mousseau J. J.; Choi C.; Bull J. A. Lithium-Catalyzed Thiol Alkylation with Tertiary and Secondary Alcohols: Synthesis of 3-Sulfanyl-Oxetane as Bioisosteres. Chem.—Eur. J. 2018, 24, 818–821. 10.1002/chem.201705576. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Dubois M. A. J.; Lazaridou A.; Choi C.; Mousseau J. J.; Bull J. A. Synthesis of 3-Aryl-3-Sulfanyl Azetidines by Iron-Catalyzed Thiol Alkylation with N-Cbz Azetidinols. J. Org. Chem. 2019, 84, 5943–5956. 10.1021/acs.joc.9b00613. [DOI] [PubMed] [Google Scholar]
- Saejong P.; Rojas J. J.; Denis C.; White A. J. P.; Voisin-Chiret A. S.; Choi C.; Bull J. A. Synthesis of Oxetane and Azetidine Ethers as Ester Isosteres by Brønsted Acid Catalysed Alkylation of Alcohols with 3-Aryl-oxetanols and 3-Aryl-azetidinols. Org. Biomol. Chem. 2023, 21, 5553–5559. 10.1039/D3OB00731F. [DOI] [PubMed] [Google Scholar]
- For a recent alternative strategy, see:; Tian D.; Chen G.; Wang X.; Zhang H.-J. Modular Access to Functionalized Oxetanes as Benzoyl Bioisosteres. J. Am. Chem. Soc. 2024, 146, 18011–18018. 10.1021/jacs.4c04504. [DOI] [PubMed] [Google Scholar]
- For approaches to thietane synthesis, see:; Xu J. Recent synthesis of thietanes. Beilstein J. Org. Chem. 2020, 16, 1357–1410. Also see ref (7) 10.3762/bjoc.16.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Baumann A. N.; Reiners F.; Juli T.; Didier D. Chemodivergent and Stereoselective Access to Fused Isoxazoline Azetidines and Thietane through [3 + 2]-Cycloaddition. Org. Lett. 2018, 20, 6736–6740. 10.1021/acs.orglett.8b02848. [DOI] [PubMed] [Google Scholar]; b Eisold M.; Müller-Deku A.; Reiners F.; Didier D. Parallel Approaches for the Funtionalization of Thietes: α-Metalation versus C-H Activation. Org. Lett. 2018, 20, 4654–4658. 10.1021/acs.orglett.8b01961. [DOI] [PubMed] [Google Scholar]; c Baumann A. N.; Reiners F.; Siegle A. F.; Mayer P.; Trapp O.; Didier D. Thiete Dioxides as Templates Towards Twisted Scaffolds and Macrocyclic Structures. Chem.—Eur. J. 2020, 26, 6029–6035. 10.1002/chem.201905751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas J. J.; Croft R. A.; Sterling A. J.; Briggs E. L.; Antermite D.; Schmitt D. C.; Blagojevic L.; Haycock P.; White A. J. P.; Duarte F.; Choi C.; Mousseau J. J.; Bull J. A. Amino-Oxetane as Amide Isosters by an Alternative Defluorosulfonylative Coupling of Sulfonyl Fluoride. Nat. Chem. 2022, 14, 160–169. 10.1038/s41557-021-00856-2. [DOI] [PubMed] [Google Scholar]
- a Dubois M. A. J.; Smith M. A.; White A. J. P.; Lee Wei Jie A.; Mousseau J. J.; Choi C.; Bull J. A. Short Synthesis of Oxetane and Azetidine 3-Aryl-3-carboxylic Acid Derivatives by Selective Furan Oxidative Cleavage. Org. Lett. 2020, 22, 5279–5283. 10.1021/acs.orglett.0c01214. [DOI] [PubMed] [Google Scholar]; b Noji M.; Sunahara H.; Tsuchiya K.; Mukai T.; Komasaka A.; Ishii K. A Novel Synthetic Route to 2-Arylalkanoic Acid by a Ruthenium-Catalyzed Chemoselective Oxidation of Furan Rings. Synthesis 2008, 2008 (23), 3835–3845. 10.1055/s-0028-1083222. [DOI] [Google Scholar]
- Love B. E.; Jones E. G. The use of Salicylaldehyde Phenylhydrazone as an Indicator for the Titration of Organometallic Reagents. J. Org. Chem. 1999, 64, 3755–3756. 10.1021/jo982433e. [DOI] [PubMed] [Google Scholar]
- Greb A.; Poh J.-S.; Greed S.; Battilocchio C.; Pasau P.; Blakemore D. C.; Ley S. V. A Versatile Route to Unstable Diazo Compounds via Oxadiazolines and Their Use in Aryl-Alkyl Cross-Coupling Reactions. Angew. Chem., Int. Ed. 2017, 56, 16602–16605. 10.1002/anie.201710445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saejong P.; Zhong J.; Rojas J. J.; White A. J. P.; Choi C.; Bull J. A.. Synthesis of 3,3-Disubstituted Thietane Dioxides [preprint]. ChemRxiv, July 22, 2024. 10.26434/chemrxiv-2024-6c9kc [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article, in its Supporting Information and openly available in the Imperial College London Research Data Repository at 10.14469/hpc/14599.