Abstract
Background
Globally, moderate wasting affects approximately 33 million children. Complex bidirectional interactions exist between wasting and infection in children. Children who experience both conditions have an increased risk of adverse outcomes including progression to severe wasting and mortality. Breaking the cycle between moderate wasting and infection could help improve growth and survival in these children. The NUTRIMAM trial will aim to investigate the efficacy of a 12-week regimen of three different nutritional interventions in at-risk young children (i.e., children who are moderately wasted and have one/more acute infections) on anthropometric recovery. Further, the study will explore whether recovery can be sustained with a post-intervention package that includes counseling and food vouchers. Sustaining anthropometric recovery beyond supplement administration will have important implications for programs.
Methods
NUTRIMAM is a multi-country, multi-center individually randomized, open-label, trial in five countries including Bangladesh, India, Mali, Pakistan, and Tanzania. A total of 6360 moderately wasted children aged 6 to 24 months with acute illness will be enrolled at health centers. Children will be randomly allocated to receive one of three dietary supplements (locally available foods, ready-to-use supplementary foods, or microbiota-directed supplementary foods) for 12 weeks. Anthropometric recovery will be assessed over this period. Participants who recover will then be re-randomized to a post-recovery support intervention comprising either counseling and food vouchers or routine standard of care for recovered children for an additional 12 weeks to determine if this intervention facilitates sustained recovery at 24 weeks.
Discussion
Children who are moderately wasted and have an infection are at higher risk of adverse outcomes. There are very few clinical trials that have been performed among children with moderate wasting with infectious illnesses to investigate if it is possible to break the undernutrition–infection cycle and thereby reduce the risk of nutritional deterioration to severe wasting or mortality and decrease the risk of acute infections. The results of the trial are anticipated to fill important evidence gaps in feeding recommendations for moderately wasted children with acute illness as well as interventions to sustain anthropometric recovery in children beyond the period of the nutritional intervention.
Trial registration
ISRCTN registry, ISRCTN53213318. Registered on April 03, 2023.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-024-08390-5.
Keywords: Childhood wasting, Nutritional intervention, Moderate acute malnutrition, Feeding recommendation, Nutritional recovery, Dietary supplements
Background
Nutrition-related factors, especially undernutrition, contributed to almost half of the 5 million deaths in children under the age of 5 in 2020 [1]. Childhood wasting (defined as weight for length/height 2 standard deviations [SD] below the median or mid-upper arm circumference (MUAC) < 125 mm) affected 6.4% of children under the age of 5 years in low- and middle-income countries in 2017. Of the estimated 45 million wasted children worldwide, 31.3 million are moderately wasted [2] (defined as weight-for-length Z score between − 2 and − 3 SD below the median based on the 2006 World Health Organization (WHO) growth standards or MUAC between 115 and 124 mm, without edema [3]). Two-thirds of these moderately wasted children live in South Asia (20 million), and nearly a third live in Africa (10 million) [4]. Moderate wasting is associated with a threefold higher risk of mortality in children under 5 years of age compared with their well-nourished peers [5]. These deaths largely occur from infectious illnesses, including diarrhea, pneumonia, malaria, and others.
While there is clear guidance on the management of children with severe wasting [6], there is currently no consistent guidance on how best to manage children with moderate wasting [7]. Most studies of different supplements report anthropometric recovery immediately on cessation of the studied supplement. Few available studies provide data on sustained recovery beyond the period of the nutritional intervention. Further, most studies reporting anthropometric recovery with different nutritional supplements are from Africa while the largest burden of moderate wasting is in South Asia. Many of these studies focused on comparisons of effectiveness between specially formulated therapeutic foods.
Evidence concerning the nutritional management of moderate wasting is limited. A Cochrane review [8] reported that the use of ready-to-use supplementary foods (RUSF) improved the recovery rate by 7% when compared to corn soy blend (CSB) (relative risk (RR): 1.07; 95% CI: 1.02 to 1.13; 6 studies; 5744 participants) in children with moderate wasting. RUSF reduced the likelihood of progression to severe wasting by 26% (RR: 0.74; 95% CI: 0.57 to 0.95; three studies; 3256 participants) compared with CSB [8]. There was no evidence of difference in recovery rate when standard RUSF was compared to local or homemade food (RR: 0.92; 95% CI: 0.64 to 1.33; 3 studies; 435 participants) [8].
A debate has emerged regarding the necessity of supplementary food (such as RUSF) for moderate wasting when a less costly and more sustainable alternative would be to use locally available food (LAF) integrated with nutritional counseling, which some evidence suggests as having a similar beneficial effect to RUSF [8, 9]. The potential advantages of LAF would be its higher acceptability, the lower likelihood of displacing the usual diet and breastfeeding, and the maintenance of high dietary diversity, all of which could lead to a sustained anthropometric recovery. However, more concrete evidence on the effectiveness of local foods needs to be assessed.
Another type of specially formulated food—microbiota-directed complementary foods (MDCF)—is formulated based on the knowledge that dysbiosis in the gut microbiota occurs in undernutrition to foster the relevant healthy microbes that facilitate repair and colonization by a more age-appropriate mature microbiota. MDCF comprises easily available ingredients and could provide an effective, affordable, culturally acceptable, and sustainable approach to treatment. MDCF-2, consumed during the complementary feeding period (6–24 months of age) by moderately wasted children, has been shown to increase the presence and functions of growth-promoting bacteria in the developing microbiota [10] and could provide an effective approach to treatment and sustained anthropometric recovery. In Bangladeshi children with moderate wasting, 3 months of MDCF compared with RUSF showed a higher rate of change in weight-for-length Z score, weight-for-age Z score, and mid-upper arm circumference [10]. Children who received MDCF had an increase in the levels of growth-related plasma proteins including mediators of bone growth, neurodevelopment, and inflammation and the abundance of 23 growth-associated bacterial taxa [10]. However, the impact of MDCF-2 (hereafter called microbiota-directed supplementary food—MDSF) on the recovery from wasting needs to be evaluated globally in comparison to other alternatives including RUSF or locally available foods.
Several studies have shown a high relapse rate among treated children once the period of active feeding has stopped [5, 11, 12], ranging from 15% [11] to 27% [5] for children who were mostly classified as having moderate acute malnutrition (MAM) at enrollment. Possible causes of relapse include ongoing household food insecurity, poor feeding practices, and/ or recurrence of infectious illnesses [13]. Direct evidence on effective interventions for sustained recovery of moderate wasting is not available. Given these reasons, counseling caregivers after recovery, in addition to ensuring adequacy of food at home, is likely to be important for optimal outcomes.
In addition to these factors, evidence from published literature indicates a higher risk of adverse outcomes in moderately wasted children with acute illnesses [14]. The relationship between malnutrition and infection is synergistic, complex, and bidirectional, each exacerbating the other. Infections aggravate malnutrition by reducing appetite, inducing catabolism, and increasing the demand for nutrients and nutrient losses. Weight loss during an infection is common when increased nutritional needs are not met by increased consumption [15, 16]. Failure to return to normal nutritional status after an illness heightens a child’s susceptibility to further infections, perpetuating a cycle of worsening nutritional status [17]. Malnourished children experience a higher incidence of infections like diarrhea or pneumonia, with increased severity and case fatality rates [18–21], identifying moderately wasted sick children at an elevated risk of death [22].
There are very few clinical trials that have been performed among children with moderate wasting with infectious illnesses to investigate if it is possible to break the undernutrition–infection cycle and thereby reduce the risk of nutritional deterioration to severe wasting or mortality and decrease the risk of acute infections. The NUTRIMAM (NUTRItional MAnagement of Moderate wasting) trial proposes to evaluate three different nutritional supplements in young children at high risk, who are moderately wasted and present with an acute infection.
Methods
The trial protocol is reported in line with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines [23].
Aims and hypotheses
The NUTRIMAM trial has the following aims
To determine the effect of (a) locally available foods (LAF) compared to ready-to-use supplementary food (RUSF) and (b) microbiota-directed supplementary food (MDSF) compared to RUSF, when given to moderately wasted children aged 6 to < 24 months presenting to a health facility with an acute illness, on anthropometric recovery from wasting within 12 weeks of enrolment. We hypothesize that supplementation with (i) LAF will be non-inferior to supplementation with RUSF and that (ii) MDSF will be superior to supplementation with RUSF, on the proportion of moderately wasted children showing initial anthropometric recovery by week 12 post-enrolment.
To determine the effects of (a) LAF compared to RUSF and (b) MDSF compared to RUSF on sustained anthropometric recovery at 24 weeks after enrolment. We hypothesize that supplementation with (i) LAF will be superior to supplementation with RUSF and that (ii) MDSF will be superior to supplementation with RUSF, on the proportion showing sustained recovery by week 24 post-enrolment.
To determine the effects of a post-recovery support intervention (continued counseling and food vouchers) compared with standard of care on sustained anthropometric recovery at 24 weeks after enrolment in the study. We hypothesize that follow-up of the recovered child including counseling and food vouchers when necessary will result in higher rates of sustained recovery at 24 weeks.
Definitions
Moderate wasting is defined as weight-for-length Z score (WLZ) < − 2 but ≥ − 3 or MUAC < 125 mm but ≥ 115 mm. Anthropometric recovery is defined as WLZ ≥ − 2.0 or MUAC ≥ 125 mm (depending on criteria of moderate wasting present at the time of enrolment).
Trial design
NUTRIMAM is a multi-country, multi-center individually randomized open-label, sequential multiple assignment, randomized trial divided into two phases.
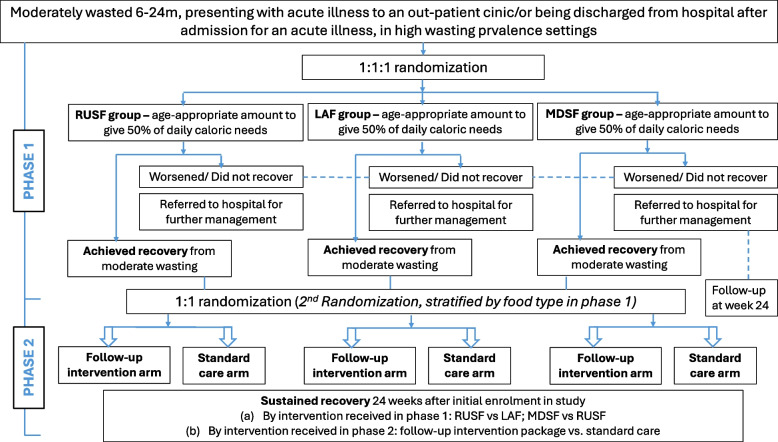
Phase 1 will test the effect of three types of food (RUSF, LAF, and MDSF) for the management of moderate wasting on anthropometric recovery within 12 weeks of enrolment. Eligible participants will be children aged 6–24 months of either sex who are moderately wasted and are diagnosed with one/more acute illnesses. Participants who meet the inclusion criteria and whose parents/guardians have provided consent will be randomized to receive either RUSF, LAF, or MDSF for the following 12 weeks. Phase 2 of the study will be focused on sustaining anthropometric recovery. Children who recover from moderate wasting in phase 1 will be randomized to receive either the post-recovery follow-up intervention (counseling and food vouchers) or standard care (national standard of care for recovered children in the country). At the end of the follow-up period (24 weeks after enrolment), weight, length, and MUAC will be measured to assess if recovery has been sustained. Figure 1 summarizes the trial design.
Fig. 1 .
Overview of trial design
Study setting
The study will be conducted in five countries: Bangladesh, India, Mali, Pakistan, and Tanzania. The specific settings within these countries are described in Table 1. These countries were chosen based on the (i) prevalence of moderate wasting, (ii) representation from geographies which bears a significant burden of wasting—Africa and South Asia, (iii) participation of countries where the results of the NUTRIMAM trial are most relevant, (iv) experience of the sites in implementing large pediatric randomized trials, (v) investigators and sites familiar with implementing community-based research in the area of child malnutrition, and (vi) investigators and sites with strong links to program and policy in the respective country.
Table 1.
Health centers by study sites
| Bangladesh | India | Mali | Pakistan | Tanzania | |
|---|---|---|---|---|---|
| OP | icddr,b, Dhaka hospital |
• CPHK, New Delhi • LLRM Medical College, Meerut, UP |
• ASACOSAB1 (Sabalibougou, commune V) • CSREF commune V • ASACOYIR (Yirimandio, commune VI) • CSREF commune VI • CHU-GT |
• Ali Akbar Shah (AKU PHC) • Machar Colony (SINA site) • Sindh Govt Ibrahim Hyderi tertiary Hospital |
• Mbagala Round Table • Temeke District Hospital |
| IP |
• CSREF commune VI • CHU-GT |
• Temeke District Hospital |
CHU-GT Gabriel Touré University Hospital, CPHK Center for Public Health Kinetics, CSCOM community health center, CSREF referral health center, icddr,b International Centre for Diarrhoeal Disease Research, IP inpatient, LLRM Lala Lajpat Rai Memorial, OP outpatient, PHC primary health center, UP Uttar Pradesh
Participants
Eligibility criteria
Inclusion criteria
Age 6 to < 24 months
Moderate wasting, defined as WLZ < − 2 but ≥ − 3 or MUAC < 125 mm but ≥ 115 mm
- Presenting at an outpatient clinic or discharged from the hospital within 7 days due to any of the following acute illnesses:
- Diarrhea, defined as three or more loose or watery stools with or without blood in the previous 24 h, and no dehydration on assessment. If the child has dehydration, the dehydration will be treated before re-assessing for recruitment.
- Pneumonia, defined as the presence of fast breathing or chest indrawing
- Non-malarial fever, defined as reported or measured (≥ 38 °C) fever of > 3 days but < 7 days duration
- Malaria, defined as reported or measured (≥ 38 °C) fever and positive rapid diagnostic test
Written informed consent for study participation from the parent or legal guardian
Exclusion criteria
Children who have any of the following will be excluded from the study:
Severe acute malnutrition (WLZ < − 3 or MUAC < 115 mm or edema of both feet)
General danger sign (lethargic/unconscious, convulsions, unable to drink, vomits everything)
Severe illness (severe pneumonia or very severe disease, severe dehydration, neck stiffness)
Persistent diarrhea (≥ 14 days)
Chronic infections/illness or disability (e.g., tuberculosis, HIV, congenital defects, cerebral palsy)
Any condition that requires hospitalization
Well child attending clinic only for routine growth monitoring or immunization
Child already receiving food supplements for moderate wasting
Treated for moderate wasting in the previous 3 months
Previously enrolled in the trial
Child with any known history of food allergy or food intolerance
Another child in the same household already recruited into the study
Child lives outside the study area routinely or will be outside of the study area for more than 2 weeks in the upcoming 3 months
Intervention
Phase 1
After obtaining a written consent from the caregivers, the child will be randomized to one of three supplements, i.e., RUSF, LAF, or MDSF daily for 12 weeks, while continuing their usual diet including breast milk. In all three intervention groups (RUSF, LAF, and MDSF), the target will be for each enrolled child to receive approximately 65 kcal/kg/day from the supplement, approximately 50% of the child’s targeted energy intake of 130 kcal/kg body weight per day to achieve catch-up growth and achieve recovery (the rationale for this energy intake is explained in Additional File 1). The remaining 65 kcal/kg body weight per day will be expected to come from the usual diet, including breastfeeding.
RUSF
Daily rations of commercially procured RUSF will be given to the caregivers along with initial counseling on how to feed this to the child. Each packet of the RUSF (100 gm) provides approximately 500 kcal, including 13 g protein and 30 g fat in addition to micronutrients (see Additional file 2, Table a). The RUSF intervention is to be given in addition to breastfeeding and complementary foods. Sufficient RUSF packets will be provided to contribute at least 65 kcal/kg/day to the infant/child’s diet.
RUSF can be given either mixed into a meal or as a snack between meals. The caregivers will be counseled on how to give the RUSF and how to maintain dietary adequacy and diversity. RUSF is complete in terms of micronutrient requirements for catch-up growth and no additional micronutrient supplements will be given. The composition will be in line with the technical specifications for RUSF issued by the World Food program in January 2021 [24].
LAF
Children randomized to this group will receive locally available foods (LAF) which will be prepared/processed in the home. The caregivers will be provided with raw ingredients to prepare food every week. These will include locally available and acceptable cereals and pulses, flour, nuts, sugar, oil, milk, fruit, and eggs (these may vary by site). Recipes for making energy-rich and nutrient-rich foods for children with locally available raw ingredients will be provided and demonstrated to the caregiver. Recipes will be designed to enhance dietary adequacy and increase dietary diversity.
A micronutrient preparation providing the recommended daily intake of vitamins and minerals for a child with moderate wasting will be given to the caregivers to be added to the cooked meal or given directly prior to feeding. The composition of the micronutrient powder will align with the UNICEF guideline on the use of multiple micronutrient powders for point-of-use fortification of foods with multiple micronutrient powders consumed by infants and young children aged 6–23 months [25].
The caregivers will receive age-appropriate LAF ingredients, enabling them to prepare between three and seven LAF recipes per site, depending on the availability of locally sourced raw materials. These recipes were selected based on the findings of formative work conducted in the study sites and a trial for improved practices, during which insights into the typical dietary patterns of the target population were gathered and various recipes were tested for feasibility and acceptability (Additional File 2, Tables b and c).
MDSF
MDSF to be used as an intervention in this trial will be similar to the MDCF recipe reported in the literature which has shown a positive relationship with biomarkers related to growth and microbiota repair (Additional file 2, Table d).
MDSF will be prepared regularly at the site from raw ingredients following standard operating procedure (SOP) provided by experts with experience in on-site preparation of MDSF. Raw ingredients will be processed by the study clinics under the guidance of the expert group in this area. The MDSF will be prepared fresh so that a caregiver receives the supplement for 3 to 5 days at a time. The MDSF preparation employed in this trial mirrors the method utilized in a previous intervention [10], with the only modification being the substitution of fresh bananas with banana powder. The banana powder is prepared on-site from fresh bananas. The processed ingredients will be packed in two different pouches, one for the dry ingredients and one for the moist ingredients (peanut paste and oil contents), and delivered to the home. The caregivers will mix the ingredients prior to feeding their children.
Criteria for discontinuing intervention
If at any point during phase 1 a child develops severe wasting (WLZ < − 3 or MUAC < 115 mm), his/her participation in the study will end and will be referred to a clinic for further clinical evaluation and treatment.
Food quantities and food sharing within the household
The intervention supplements for the child enrolled in the study will be given in addition to breastfeeding and usual home foods consumed by the child. The amount of study supplement provided to the caregiver will be sufficient to allow for (i) 50% of the required daily caloric intake to be consumed by the infant/ child according to weight, (ii) wastage during feeding of index infant/child, and (iii) potential sharing within the family. To account for potential sharing within the household, each sibling under 5 years of age will be provided with half the daily supplement portion given to the study participant throughout the intervention period. These will be packed separately with clear instructions for the caregiver.
Phase 2
Children who achieve anthropometric recovery in phase 1 will be randomized again (second randomization) at the end of week 12 to one of two arms—(i) follow-up intervention package consisting of provision of nutritional counseling and food vouchers or (ii) standard care consisting of the national standard of care for children recovered from moderate wasting. Phase 2 will be initiated 12 weeks after enrolment and will continue until the end of 24 weeks after initial enrolment. At the start of phase 2, the caregivers will be counseled by the trained staff that their children, now having gained enough weight and recovered, do not need the phase 1 food supplements anymore, and should eat only home foods. However, attention needs to be given to the amount and quality of the child’s diet, so that recovery can be sustained. Counseling will focus on how this can be achieved, with continued breastfeeding and home foods, including frequency and amounts of food, diversity of foods, and frequent handwashing. Mothers will be informed of danger signs which signal that the child needs to return to the facility.
Arm 1: post-recovery intervention package
Children randomized to this arm will receive nutritional counseling and food vouchers, while continuing to receive routine age-appropriate program care as per national guidelines for this age group (e.g., immunization)—as described below:
-
(i)
Counseling: on enrolment into phase 2, the mother/caregiver will be counseled on continued attention to feeding practices, encouraged to reinforce these practices, and given information on danger signs of illness in the child.
-
(ii)
Food vouchers: alongside counseling, the mothers/caregivers will receive three food vouchers at the time of the second randomization. These food vouchers can be redeemed once per month for food ingredients from pre-specified vendors/clinic sites that will accept the vouchers in exchange for a pre-selected list of ingredients (e.g., oil, sugar, nuts, flour, fruit, milk, eggs). The caregivers will have the flexibility to select items from different food categories (carbohydrates, protein, and fats/oils), ensuring that they choose at least one item from each category. Each site will tailor the options for each category of food based on local availability and appropriateness for the age of the index child; the only stipulation provided is that each food voucher should cover the cost of providing 750 kcal per day for the index child, with the total calories evenly distributed among the three food categories. To exercise agency, the caregivers must personally choose and collect the food items.
Arm 2: standard care during phase 2
Children in this arm will receive the national standard of care for children recovered from moderate wasting. The national standard of care is similar across the sites with minor variations, summarized in Table 2.
Table 2.
National standards of care following recovery from MAM
| Country | National standard of care following recovery from MAM |
|---|---|
| Bangladesh | There is no guideline or prescribed follow-up or active standard of care for children who have recovered from MAM in Bangladesh. |
| India | There is no guideline or standard follow-up for child post recovery from MAM |
| Mali | There is no such official standard of care in Mali. However, the usual practice in the study health centers to follow a child after recovery from MAM once a month for 3 months for nutritional status by measuring MUAC, weight, and length/height. This is compatible with trial participation and follow-up. |
| Pakistan | As per CMAM National guidelines, the child who recovers from MAM should receive dietary counseling for complementary feeding and continuation of breastfeeding till 2 years of age. This is compatible with follow-up during the trial. |
| Tanzania | On recovery from MAM, a continuum of care is advised by following the child at the primary health clinic and where available to attend special nutritional clinics. These nutrition clinics provide counseling and measurement of anthropometry. They are supposed to provide supportive services as well. This is compatible with participation in the NUTRIMAM trial |
Outcomes
Primary outcomes
The primary outcome for phase 1 of the trial is the proportion (%) of participants who have an anthropometric recovery from moderate wasting at any point up to 12 weeks after enrolment. Anthropometric recovery will be defined as WLZ ≥ − 2.0 or MUAC ≥ 125 mm, based on their baseline enrollment criteria. For children enrolled on the basis of WLZ alone, recovery will be when the child attains WLZ greater than or equal to − 2.0. For those enrolled solely according to MUAC, recovery will be when the child’s MUAC is equal to or exceeds 125 mm. For children meeting both criteria upon enrollment, reaching either a WLZ greater than or equal to − 2.0 or a MUAC of 125 mm or more will be considered as recovery. This primary outcome will be compared between (a) LAF vs RUSF groups and (b) MDSF vs RUSF groups.
The primary outcome for phase 2 of the trial is the proportion (%) of participants who sustain anthropometric recovery at 24 weeks, defined as meeting the 12-week anthropometric outcome by 12 weeks and maintaining anthropometric recovery at 24 weeks. If a child was enrolled based solely on WLZ criteria, sustained recovery will entail having a WLZ ≥ − 2.0 by 12 weeks and maintaining WLZ ≥ − 2.0 at the 24-week visit. If a child was enrolled based solely on MUAC criteria (MUAC < 125 mm but ≥ 115 mm), sustained recovery is defined by achieving a MUAC ≥ 125 mm by 12 weeks and maintaining MUAC ≥ 125 mm at the 24-week visit. For children enrolled based on both WLZ and MUAC criteria, sustained recovery is achieved when the child has either WLZ ≥ − 2.0 or MUAC ≥ 125 mm by 12 weeks and maintains either WLZ ≥ − 2.0 or MUAC ≥ 125 mm at the 24-week visit. We will compare this primary outcome between (1) (a) LAF vs RUSF groups and (b) MDSF vs RUSF groups and (2) follow-up intervention package vs standard care.
Secondary outcomes
Median time to recovery between the three groups within the first 12 weeks
- Mean change in (i) mid-upper arm circumference (MUAC, in mm), (ii) weight-for-length Z score (WLZ), (iii) weight-for-age z-score (WAZ), and (iv) length-for-age Z score (LAZ):
- Between MDSF, LAF, and RUSF from (i) enrolment to 12 weeks and (ii) enrolment to 24 weeks
- Between food voucher and standard care post-recovery support groups from week 12 to week 24
- Mean change in skinfold thickness measurements:
- Between MDSF, LAF, and RUSF, from (i) enrolment to 12 weeks and (ii) enrolment to 24 weeks
- Between voucher and standard care post-recovery support groups from week 12 to week 24
- Deterioration to SAM or death:
- Between MDSF, LAF, and RUSF from (i) enrolment to 12 weeks and (ii) enrolment to 24 weeks
- Between voucher and standard care post-recovery support groups from week 12 to week 24
- All-cause mortality:
- Between MDSF, LAF, and RUSF from (i) enrolment to 12 weeks and ii) enrolment to 24 weeks
- Between voucher and standard care post-recovery support groups from week 12 to week 24
- Any hospitalization (> 24 h):
- Between MDSF, LAF, and RUSF from (i) enrolment to 12 weeks and (ii) enrolment to 24 weeks
- Between voucher and standard care post-recovery support groups from week 12 to week 24
- Deterioration to SAM:
- Between MDSF, LAF, and RUSF from (i) enrolment to 12 weeks and (ii) enrolment to 24 weeks
- Between voucher and standard care post-recovery support groups from week 12 to week 24
Additional exploratory outcomes
We will also perform exploratory analysis of MDSF compared to LAF for all primary and secondary outcomes.
Participant timeline
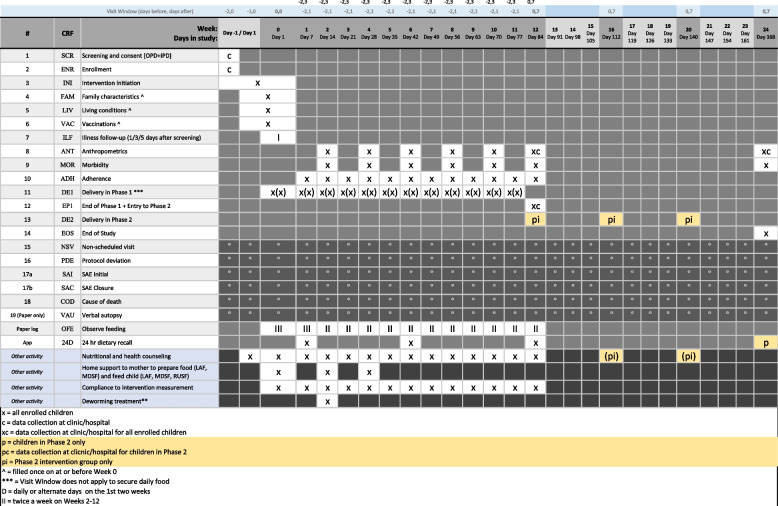
Screening, informed consent, and randomization will take place in study clinics/hospitals where the children have been brought for illness care. Randomized children will receive food deliveries and scheduled follow-up visits (adherence, anthropometric, and morbidity) in their household during phase 1 of the intervention at regular intervals until week 12, regardless of recovery status. Children who recovered from moderate wasting at any point during the first 12 weeks will be eligible to enter phase 2 at the end of week 12 and will be rerandomized into the post-recovery support intervention or the standard care arm for the following 3 months. At the end of the phase 2 follow-up period, 24 weeks after enrolment, weight, length, and MUAC will be measured in all children to assess if recovery has been sustained (Figs. 1 and 2).
Fig. 2.
Schedule of enrolment, interventions, and assessments
Given the burden of moderate wasting among young children seen at the recruiting sites, the estimated recruitment rate will be 200–250 participants (40–50 per site). The overall duration from the initiation of recruitment to the last follow-up is expected to take 30 months.
Screening, informed consent, and recruitment to the trial
Screening will be conducted at two places in the recruiting health centers (i) at the outpatient clinics at presentation and (ii) at inpatient pediatric departments at the time of discharge. Screening will be done by the trained research team members at each recruiting facility. Broadly, the screening process will involve a confirmation of the child’s age, the nutritional status of the child, and details of the child’s illness. A screening form will allow the information collected to be matched against inclusion and exclusion criteria to determine if the child is eligible for recruitment. No identifying details will be collected at the time of screening. If the child has any danger sign, the screening will be paused and the child will be given emergency medical attention.
If on the completion of the screening process the child is eligible, then the written informed consent from the primary caregiver will be sought (Additional File 4).
Children who meet all the inclusion criteria and none of the exclusion criteria and whose parents/legal guardians have provided consent will be recruited into the trial. The participants will first be provided with initial case management for their acute illness in accordance with the standard of care by medically trained personnel. The caregivers will be interviewed to collect data on sociodemographic characteristics and other baseline characteristics of the child. The caregiver will be provided with detailed information of the study procedures, and visit schedule and a suitable time when the study team can visit the caregiver and child in the home will be agreed upon. The child will be randomized to one of the three study arms and details of the relevant intervention provided. The caregiver will also be given information about the dates of supplement delivery and methods of feeding the supplements. The first home visit will be scheduled within 48 h of enrollment, during which the study staff will discuss and demonstrate the preparation and feeding of the supplement.
Allocation sequence generation
Allocation to intervention groups will be concealed through the use of a web interface based on a computer-generated randomization sequence. Randomization of children into phase 1 will occur once eligibility and consent have been confirmed. Participants in phase 1 will be randomly assigned to LAF, MDSF, or RUSF in a 1:1:1 allocation ratio. Blocked randomization with varying block sizes will be performed to reduce predictability. Randomization of eligible children (anthropometrically recovered) into phase 2 will be done at the end of week 12, just at the beginning of phase 2. For phase 2, stratified randomization by intervention group in phase 1 will be done in blocks of 6. The randomization sequences will be generated through a web interface.
Blinding
Given the nature of the interventions, participants and their caregivers will not be blinded to the study arm. However, a separate team of research staff to measure outcomes (anthropometry) will be designated at each site to facilitate the blinding of outcome measurements to the extent possible in an open-label trial of this nature. The analysis will be conducted in a blinded manner, ensuring that the study arms remain masked throughout the analysis process.
Retention and follow-up procedures
Upon randomization at the facility, we will collect contact information—including addresses, phone numbers, and relatives' details of all participants to ensure effective community follow-up. After enrollment, the caregivers will be provided with detailed information (both written and verbal) about the intervention assigned to their child. They will also be informed that data collectors will come to the household to complete (i) weekly intervention adherence and feeding observation visits and fortnightly, (ii) anthropometric, and (iii) morbidity recording visits during phase 1. Data collectors will make every reasonable effort to follow the child through the entire study period.
Relevant concomitant care permitted during the trial
Initial management of the identified episodes of illness will be identical in all three treatment groups and performed according to standard Integrated Management of Childhood Illness (IMCI) management guidelines [26]. Site-specific adaptations to these IMCI guidelines in line with national guidelines will be permissible.
Feeding instructions and nutrition counseling
The caregiver will be advised to maintain the child’s regular diet and continue breastfeeding in addition to the study supplement. He/she will also be informed about the duration of the intervention, how frequently the food will be delivered, and how it will be packaged. Confirmation of recipes in case of LAF will be provided, along with instructions on preparing, mixing, or cooking the supplement before feeding it to the child. Additionally, the caregivers will be informed about the extra food given for the other under-5 children in the home and how to differentiate food for the index child and other siblings. They will be made aware that a local observer will visit weekly to offer support, if needed. The caregivers will be expected to record the child’s daily supplement consumption on standardized pictorial cards, which will be collected weekly. The caregivers will also be provided with the phone number of the research staff to contact them at any point in the study.
Nutrition counseling will be conducted frequently in the first week and then weekly from weeks 2 to 4. Thereafter, this will be done fortnightly up to week 12, if the child is improving. If the child is improving at any point, then the counseling frequency will be adapted accordingly. The content of the counseling will vary depending on the growth trajectory that the child is experiencing. The content of counseling will be in line with the WHO module on counseling on growth and feeding [27].
Intervention adherence
Measuring the actual intake of nutrition interventions or “sharing” among other members of the household is a challenge for most nutrition intervention studies. Acceptability of and compliance to the intervention are important determinants of the effectiveness of the interventions and their ascertainment is important to explain why one intervention is more or less effective than the other. In this study, adherence will be measured based on pictorial cards filled in by the caregivers daily that record the amount of supplement consumed and collected once a week by the data collectors during a home visit.
Anthropometric and morbidity visits
For the duration of phase 1, anthropometric data will be collected fortnightly to provide information on recovery. Morbidity data will also be recorded.
Data collection methods
Data capture will be electronic with REDCap serving as the database.
Data management
Data will be managed centrally by a clinical trials data management team, supervised directly by the WHO Trial Coordinating Unit (TCU). The study statistician will be responsible for overseeing data management, as well as the development of the statistical analysis plan and execution of the pre-planned data analysis and any subsequent secondary analyses. Quality control will be performed at each site. Each site will have a data manager responsible for local query management and ensuring case report forms (CRFs) are correctly filled out. Any discrepancies will be immediately addressed. All data stored in the database will be anonymized with no identifying details.
Confidentiality
The following measures will be taken to ensure participant confidentiality:
Trial data for each participant will be identified by a unique study ID number.
The local trial register linking personal information and trial ID numbers, and all personal information of participants, will be kept separate from the CRFs.
Trial documents will be kept securely under lock and key in the research offices and will not be accessible, other than the researchers.
Data will be entered by trial ID number in the password-protected data management system to which only trial staff will have access.
The trial report will not contain the names of any participants.
Statistical methods
Sample size and power calculations
A total of 6360 children (i.e., 2120 per arm) will be randomized in a 1:1:1 ratio to the three arms (RUSF, LAF, and MDSF) at the beginning of phase 1. Sample size calculations were based on the LAF vs RUSF non-inferiority comparison since it required the largest sample size. We accounted for multiple comparisons for each pairwise arm comparison at 12 and 24 months (e.g., there are two tests for LAF vs RUSF). For the 12-week anthropometric recovery, we will make two primary comparisons: MDSF vs RUSF and LAF vs RUSF, and therefore, we have used an α = 0.025 (0.05/2 tests) in power calculations. We similarly used an α = 0.025 for power calculations for two primary comparisons of MDSF vs RUSF and LAF vs RUSF on the sustained recovery outcome at 24 weeks. There is only one test for the sustained recovery intervention as compared to the control group and therefore an α = 0.05 was used.
Primary comparison #1: LAF vs RUSF on 12-week anthropometric recovery (non-inferiority test)
We anticipate that 60% of enrolled children will achieve anthropometric recovery within 12 weeks of enrolment in both RUSF and LAF groups. Average recovery rates in children receiving RUSF or corn soy blend is reported to be about 70% in studies included in a systematic review by Das et al. [8]. We have chosen a more conservative estimate of 60% allowing for a lower recovery rate than that previously reported (slightly lower rates have been reported in South Asian contexts). The non-inferiority margin was set at an absolute risk difference of 5% based on the judgment of clinical and public health importance of differences in primary outcome. We also assumed a one-sided α = 0.025, 90% power, and 5% loss to follow-up, which resulted in 2120 participants required in each group.
Primary comparison #2: MDSF vs RUSF on 12-week anthropometric recovery (superiority)
This hypothesis is based on the evidence from a proof of principle study conducted in Bangladesh which showed that MDSF is better than RUSF in promoting growth. We anticipate that 60% of enrolled children will achieve anthropometric recovery within 12 weeks of enrolment in the RUSF group (comparison group). Based on an enrollment of 2120 per group, 1:1 randomization, an α = 0.025, and 5% loss to follow-up/missing data, we will have 90% power to detect a relative risk of 1.09 for 12-week anthropometric recovery for MSDF as compared to RUSF.
Primary comparison #3: LAF vs RUSF on 24-week sustained anthropometric recovery (superiority)
The LAF group may have a greater proportion of children with sustained recovery than RUSF because it has higher acceptability, lower likelihood of displacing usual diet and breastfeeding, and an easier transition to giving adequate home foods to the child in phase 2 after recovery has been achieved. We anticipate that overall, 24% of children will achieve 24-week sustained anthropometric recovery in the RUSF group (comparison group). Based on an enrollment of 2120 per arm, 1:1 randomization, an α of 0.025, and 10% loss to follow-up/missing data, we will have 90% power to detect a relative risk of 1.21 for 24-week sustained anthropometric recovery for LAF as compared to RUSF.
Primary comparison #4: MDSF vs RUSF on 24-week sustained anthropometric recovery (superiority)
This hypothesis is also based on the evidence from a proof of principle study conducted in Bangladesh which showed that MDSF is better than RUSF in promoting growth. We anticipate that 40% of enrolled children who recovered in phase 1 (assumed 60% initial recovery) will have sustained recovery, which amounts to 24% of all children randomized having achieved 24-week sustained anthropometric recovery in the RUSF group (comparison group). Based on an enrollment of 2120 per arm, 1:1 randomization, an α of 0.025, and 10% loss to follow-up/missing data, we will have 90% power to detect a relative risk of 1.21 for 24-week sustained anthropometric recovery for MSDF as compared to RUSF.
Primary comparison #5: sustained recovery intervention vs standard of care on 24-week sustained anthropometric recovery (superiority)
We assume that 60% of the participants would achieve 12-week anthropometric recovery and are therefore included in the second-stage randomization (n = 1272 in each arm are estimated to undergo second-phase randomization). We assume that 45% in the standard-of-care group will have 24-week sustained anthropometric recovery. Based on an enrollment of 1272 per arm, 1:1 randomization, an α = 0.05, and 5% loss to follow-up/missing data, we will have 90% power to detect a relative risk of 1.15 for 24-week sustained anthropometric recovery for the sustained recovery intervention as compared to standard of care.
Statistical methods
An intent-to-treat (ITT) analysis will be the primary approach for all comparisons of primary and secondary outcomes, i.e., all randomized children to be included in the analysis. Due to stratified randomization by study site, we will include a fixed effect for the study site in all models. A per-protocol analysis will also be performed for comparisons between MDSF vs RUSF and LAF vs RUSF. The per-protocol analyses will only include participants whose mother/caregiver reported the child consumed the food supplement for at least 75% of the days between randomization and 12 weeks and have data for the outcome of interest at 12 weeks for the initial recovery outcome or 24 weeks for the sustained recovery outcome. Per-protocol analyses are not planned for the second-stage interventions due to the use of a control group in which adherence does not apply.
Primary comparisons
Primary comparison #1: LAF vs RUSF on 12-week anthropometric recovery (non-inferiority test)
We will use generalized linear models with a binomial distribution and identity link function to produce risk difference estimates for the LAF arm compared to the RUSF arm. The lower bound of the one-sided 97.5% confidence interval (to account for multiple comparisons) of the risk difference arm will be compared with the prespecified noninferiority margin of − 5% to assess non-inferiority. To allow for direct comparison to other studies and inclusion in meta-analyses, we will also present relative risk estimates with standard two-sided 95% confidence intervals.
Primary comparison #2: MDSF vs RUSF on 12-week anthropometric recovery (superiority)
We will use a log-binomial regression model to present the relative risk (RR) of 12-week anthropometric recovery for the MDSF arm as compared to the RUSF arm. We will present the relative risk, two-sided 97.5% confidence intervals, and a p-value < 0.025 will be considered statistically significant to account for multiple comparisons.
Primary comparison #3: LAF vs RUSF on 24-week sustained anthropometric recovery (superiority)
We will use a log-binomial regression model to present the RR of 24-week sustained recovery for the MDSF arm as compared to the RUSF arm. We will present the relative risk, two-sided 97.5% confidence intervals, and a p-value < 0.025 will be considered statistically significant to account for multiple comparisons.
Primary comparison #4: MDSF vs RUSF on 24-week sustained anthropometric recovery (superiority)
We will use a log-binomial regression model to present the RR of 24-week sustained recovery for the MDSF arm as compared to the RUSF arm. We will present the relative risk, two-sided 97.5% confidence intervals, and a p-value < 0.025 will be considered statistically significant to account for multiple comparisons.
Primary comparison #5: sustained recovery intervention vs standard of care on 24-week sustained anthropometric recovery (superiority)
We will use a log-binomial regression model to present the RR of 24-week sustained anthropometric recovery for the sustained recovery intervention as compared to the standard of care arms. We will present the relative risk, 95% confidence intervals, and a p-value < 0.05 will be considered statistically significant.
Secondary outcomes
The following analytic methods will be used for secondary outcome analyses. For time-to-event analyses, we will use Cox proportional hazard regression with time as follow-up time in days since randomization to produce hazard ratios and 95% confidence intervals. For changes in anthropometric measure outcomes (e.g., change in WLZ), we will use generalized linear models that also include a covariate for the baseline measure to produce mean differences and 95% confidence intervals. For binomial outcomes (e.g., mortality), we will use log-binomial regression models to present the relative risks and 95% confidence intervals.
Sensitivity analyses for potential baseline imbalance
In sensitivity analyses for the primary outcomes to assess the potential for baseline imbalance to affect results, we will adjust for baseline factors that showed some degree of imbalance between treatment groups based on a p < 0.10. We will report the results of these sensitivity analyses and discuss if there are any qualitative differences in the findings of the baseline-adjusted and the primary unadjusted analyses.
Exploratory analyses for potential effect modifiers
We will explore the potential effect modification of treatment effects for only the primary comparisons. We have a priori defined the following potential effect modifiers:
Site region (South Asia (Bangladesh, India, Pakistan) vs sub-Saharan Africa (Tanzania and Mali))
Diarrhea at enrollment (yes vs no)
Participant age at enrollment (6 to < 12.0 months vs 12.0 to 24 months)
Participant sex (male vs female)
Moderate acute malnutrition (MAM) diagnosis type at enrollment (WLZ only, MUAC only, both WLZ and MUAC)
Due to the exploratory nature of these analyses, we will not adjust for multiple comparisons but rather recognize there is a risk of type II error. The same modeling approaches will be used as the primary comparisons but these analyses will include interaction terms between treatment and the effect modifier of interest. The statistical significance of effect modification will be assessed with the likelihood ratio test.
Missing data
We will assume that all outcome data are missing completely at random and employ a complete case analysis as the primary analytic strategy. This means that only participants with data for the outcome of interest will be included in the analysis. Specifically, participants who do not complete any follow-up visits by the 12-week mark will be excluded from the analysis. Additionally, individuals who achieve anthropometric recovery at 12 weeks but lack follow-up data at 24 weeks—due to loss to follow-up, missing data, death, or withdrawal—will also be excluded. We will not impute values for missing outcome data.
Adverse events
Children enrolled in the study are at high baseline risk of complications as a result of their moderate wasting in the setting of acute illness. The interventions under study are safe and appropriate for the nutritional management of moderate wasting, and all participants will receive standard of care for their acute illness. The following will be considered serious adverse events: anaphylaxis, hospitalization for more than 24 h, and death. All suspected adverse events will be monitored by the site principal investigators (PIs) who will review and report these to the local Ethics Committee and to the WHO. The WHO TCU will report these to the WHO Research Ethics Committee. The Data Safety and Monitoring Board (DSMB) will also receive the information of the adverse events.
Trial oversight
Several monitoring procedures are included in this trial which have been prepared in accordance with the ICH harmonized tripartite guideline for Good Clinical Practice [28]. WHO staff will prepare a set of standard operating procedures (SOPs) for all monitoring activities which will govern all monitoring procedures.
Prior to the commencement of recruitment, WHO staff will conduct in-person training workshops with all study site research teams, in relation to screening, informed consent, recruitment, data collection, follow-up, and other study procedures. This training will emphasize the need for accurate and thorough data reporting, and vigilance in identifying, detecting, and reporting any possible adverse events, safety concerns, or protocol deviations.
Three types of monitoring visits will occur: (1) WHO will identify and recruit a group of independent trial monitors to conduct regular monitoring visits twice a year, (2) principal investigators and co-investigators will conduct regular monitoring visits to all participating clinics/hospitals to ensure compliance with trial protocol and SOPs, and (3) WHO staff will perform one to two visits annually per site.
Day-to-day oversight of the trial will be provided by the Trial Steering Committee, comprising the WHO TCU and all site principal investigators. The WHO TCU will meet monthly with each site to monitor progress. An independent DSMB will be established, composed of five members, including an independent chair, a statistician, and three technical experts familiar with nutritional studies in low-income settings and clinical trial methodology. The DSMB will monitor adverse events on a regular basis and advise the TCU accordingly.
Ethical considerations
The trial protocol was reviewed and approved by the WHO Ethics Review Committee (V.0.161 as of 29 November 2023). All participating sites received approval from the relevant institutional scientific and ethical review committees in the respective country (as well as required permissions from the relevant national regulatory authorities). Any modifications to the protocol which may impact the conduct of the study, potential benefit of the study participants or their safety, including changes to study objectives, study design, study population, sample sizes, study procedures, or other significant aspects will require a formal amendment to the protocol. The study co-investigators will agree on these amendments and submit them to the WHO Ethical Review Committee (ERC) and other relevant local ethical review boards prior to implementation.
Discussion
The current IMCI guidelines on feeding of children with moderate wasting presenting to a health facility with illness recommend counseling of the caregiver on increased feeding using home-available foods. The Essential Nutrition Actions document recommends the provision of increased amount of food after an acute illness [29], optimally locally available foods. The recent WHO guidelines [30] recommend that moderately wasted children under 5 should have access to a nutrient-dense diet to fully meet their extra needs for the recovery of weight and height and for improved survival, health, and development.
The findings from the NUTRIMAM trial are expected to fill important gaps with regard to guidance on feeding of children at risk, i.e., those with moderate wasting and an acute infection. A future sub-study to understand the effects of the different supplements on body composition, gut microbiota, proteins associated with growth, and inflammatory markers is also currently being planned.
Although factors that contribute to the increased risk of adverse outcomes in moderately wasted children are still being discussed, an active infection in a moderately wasted child clearly increases the risk of deterioration to SAM/death. There is a lack of evidence addressing various aspects such as identifying suitable interventions to effectively and sustainably recover children at high risk, determining optimal treatment durations, and establishing what would be an acceptable level of reduction in the risk of adverse outcomes from treatment strategies [31]. The NUTRIMAM trial will seek to bridge these gaps by exploring an optimal intervention for moderately wasted children at risk because of an infection, providing important evidence that will contribute to a risk-stratified approach to the management of wasting.
Trial status
Recruitment for the NUTRIMAM trial started in April 2023 and is currently ongoing (expected to be completed by December 2026). This manuscript is based on the current version of the protocol, version 0.161, which was approved by the WHO Ethics Review Committee on the 29th of November 2023.
Dissemination plan
The dissemination plan will target two main audiences:
Policy makers, program managers, and health workers: trial results will be shared with local health managers, regional and national health departments, and related agencies. Presentations at national, regional, and international conferences will also be utilized for dissemination.
Study populations: a meeting will be conducted with community leaders, local health system staff, and community health workers to debrief about the study results and express gratitude for their support. Study participants will be informed of the results and their implications through community health workers or research team members, either in group sessions or individually, tailored to the context of each site.
Supplementary Information
Additional file 1. Rationale behind targeted energy intake of 130 kcal/kg body weight per day.
Additional file 2. Food supplements composition.
Acknowledgements
The NUTRIMAM team would like to thank the children and families that have participated in the trial to date. All the staff at all the participating sites are acknowledged for their dedication. The authors also appreciate the independent oversight provided by the DSMB members (Alan Jackson, Fyezah Jehan, Kenneth Maleta, Max Petzold) and external reviewers (Per Ashorn, Nita Bhandari, Mark Manary, Jose Martinez, Jonathan Wells).
Authors
The NUTRIMAM Study Team (listed below)
| First name | Middle name | Family name | Affiliation | |
|---|---|---|---|---|
| Ishita | Mostafa | ishita.mostafa@icddrb.org | International Centre for Diarrheal Disease Research, Bangladesh (icddr, b), Dhaka, Bangladesh | |
| Jafrin | Ferdous | Jafrin.ferdous@icddrb.org | International Centre for Diarrheal Disease Research, Bangladesh (icddr, b), Dhaka, Bangladesh | |
| Irin | Parvin | irin.parvin@icddrb.org | International Centre for Diarrheal Disease Research, Bangladesh (icddr, b), Dhaka, Bangladesh | |
| Hasan | Hafizur | Rahman | Hasan.Hafizur@icddrb.org | International Centre for Diarrheal Disease Research, Bangladesh (icddr, b), Dhaka, Bangladesh |
| Kazi | Nazmus | Saqeeb | drkazisaqeeb@icddrb.org | International Centre for Diarrheal Disease Research, Bangladesh (icddr, b), Dhaka, Bangladesh |
| Mohammod | Jobayer | Chisti | chisti@icddrb.org | International Centre for Diarrheal Disease Research, Bangladesh (icddr, b), Dhaka, Bangladesh |
| Tahmeed | Ahmed | tahmeed@icddrb.org | International Centre for Diarrheal Disease Research, Bangladesh (icddr, b) Dhaka, Bangladesh | |
| Karim | Manji | kpmanji@gmail.com | Muhimbili University of Health and Allied Sciences (MUHAS), Department of Pediatrics and Child Health, Dar es Salaam, Tanzania | |
| Christopher | P | Duggan | Christopher.duggan@childrens.harvard.edu | Boston Children’s Hospital, Boston, MA, USA |
| Christopher | Sudfeld | csudfeld@hsph.harvard.edu | Harvard TH Chan School of Public Health, Boston, MA, USA | |
| Sarah | Somji | sarahsomji@gmail.com | Department of Paediatrics and Child Health, Muhimbili University of Health and Allied Sciences (MUHAS), Dar es Salaam, Tanzania | |
| Rodrick | Kisenge | saroriki@yahoo.com | Department of Paediatrics and Child Health, Muhimbili University of Health and Allied Sciences (MUHAS), Dar es Salaam, Tanzania | |
| Saidah | Mohamed Bakar | Saidah.bakar@muhas.ac.tz | Department of community health, Muhimbili University of Health and Allied Sciences (MUHAS), Dar es Salaam, Tanzania | |
| Mohamed | Bakari Kheri | mudybakari@gmail.com | Department of Paediatrics and Child Health, Muhimbili University of Health and Allied Sciences (MUHAS), Dar es Salaam, Tanzania | |
| Karen | L | Kotloff | Kkotloff@som.umaryland.edu | Department of Pediatrics and Medicine, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, MD, USA |
| Samba | O | Sow | ssow@som.umaryland.edu | Centre pour le Développement des Vaccins (CVD-Mali), Bamako, Mali |
| Dilruba | Nasrin | dnasrin@som.umaryland.edu | Department of Medicine, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, MD, USA | |
| Milagritos | Tapia | mtapia@som.umaryland.edu | Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA | |
| Adama | M | Keita | akeita@cvd-mali.org | Centre pour le Développement des Vaccins (CVD-Mali), Bamako, Mali |
| Fadima | C | Haidara | fhaidara@cvd-mali.org | Centre pour le Développement des Vaccins (CVD-Mali), Bamako, Mali |
| Jane | Jauma | jjuma@cvd-mali.org | Centre pour le Développement des Vaccins (CVD-Mali), Bamako, Mali | |
| Farah | Naz | Qamar | Farah.qamar@aku.edu | Department of Pediatric and Child Heath, Aga Khan University, Karachi, Pakistan |
| Tahir | Yusufzai | tahir.yousafzai@aku.edu | Department of Pediatric and Child Heath, Aga Khan University, Karachi, Pakistan | |
| Sabeen | Siddiqui | sabeen.siddiqui@aku.edu | Department of Pediatric and Child Heath, Aga Khan University, Karachi, Pakistan | |
| Naveed | Ahmed | Naveed.ahmed@aku.edu | Department of Pediatric and Child Heath, Aga Khan University, Karachi, Pakistan | |
| Haobijam | Prabhabati | Devi | prabhadavid29@gmail.com | Center for Public Health Kinetics, New Delhi, India |
| Priyanka | Mandal | mandalpriyanka116@gmail.com | Center for Public Health Kinetics, New Delhi, India | |
| Usha | Dhingra | udhingra@cphealthkinetics.org | Center for Public Health Kinetics, New Delhi, India | |
| Srashti | Sharma | srashti24sharma@gmail.com | Center for Public Health Kinetics, New Delhi, India | |
| Nav | Ratan Kumar | Gupta | navratankumargupta@gmail.com | LLRM Medical College Meerut, India |
| Arup | Dutta | adutta@cphealthkinetics.org | Center for Public Health Kinetics, New Delhi, India | |
| Jitendra | Kumar | admin@cphealthkinetics.org | Center for Public Health Kinetics, New Delhi, India | |
| Sunil | Sazawal | ssazawal@jhu.edu | Center for Public Health Kinetics, New Delhi, India | |
| Ayesha | De Costa* | deay@who.int | Department of Maternal, Newborn, Child and Adolescent Health, and Ageing, World Health Organization, Avenue Appia 20, Geneva, Switzerland | |
| Ameena | Goga | gogaa@who.int | Department of Maternal, Newborn, Child and Adolescent Health, and Ageing, World Health Organization, Avenue Appia 20, Geneva, Switzerland | |
| Agnese | Iuliano | iulianoa@who.int | Department of Maternal, Newborn, Child and Adolescent Health, and Ageing, World Health Organization, Avenue Appia 20, Geneva, Switzerland | |
| Nigel | Rollins | rollinsn@who.int | Department of Maternal, Newborn, Child and Adolescent Health, and Ageing,, World Health Organization, Avenue Appia 20, Geneva, Switzerland | |
| Hiresh | Tiwary | hiresh.tiwary@sas.org.in | Department of Maternal, Newborn, Child and Adolescent Health, and Ageing,, World Health Organization, Avenue Appia 20, Geneva, Switzerland |
Abbreviations
- CRF
Case report form
- DSMB
Data Safety and Monitoring Board
- ERC
Ethical review committee
- IMCI
Integrated Management of Childhood Illness
- LAF
Locally available food
- MAM
Moderate acute malnutrition
- MDSF
Microbiota-directed supplementary food
- MUAC
Mid-upper arm circumference
- NUTRIMAM
Nutritional management of moderate wasting
- RR
Relative risk
- RUSF
Ready to use supplementary food
- SOP
Standard operating procedure
- TCU
Trial coordinating unit
- WHO
World Health Organization
- WLZ
Weight-for-length Z-score
Authors’ contributions
All named authors adhere to the authorship guidelines of Trials. ADC, AG, NR, RB, IM, KM, CPD, KLK, SSo, DN, FNQ, TY, and SSa conceived the study and led the proposal and protocol development. All the other authors contributed to the development of the protocol and co-authored the manuscript. All authors read and approved the final manuscript.
Funding
This trial is supported by the Bill and Melinda Gates Foundation (grant no: INV004260). The funder had no role in study design; collection, management, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication. The trial was coordinated by the Department of Maternal, Newborn, Child and Adolescent Health and Ageing, World Health Organization, Geneva.
Availability of data and materials
The datasets generated during the current study will be available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The NUTRIMAM protocol has been approved by the WHO Ethics Review Committee and by the Ethics Committees of all participating sites in the five countries—Bangladesh (ERC of the International Centre for Diarrhoeal Disease Research), India (Institutional Ethics Committee, Subharti Medical College & Hospital), Mali (Universite Des Sciences, Des Techniques et des technologies de Bamako), Pakistan (The Aga Khan University Ethical Review Board), Tanzania (Muhimbili University of Health and Allied Sciences Senate Research and Publications Committee), and USA (Boston Children’s Hospital Institutional Review Board and University of Maryland). Each updated version of the approved protocol will be submitted to the ethical committees above.
Written informed consent (from parents/caregivers) is obtained by study staff before any trial procedures are carried out and participant confidentiality is maintained throughout the trial in line with the standard ICH-GCP principles.
Consent for publication
The members of the WHO NUTRIMAM Trials Collaboration consent to publication.
Competing interests
WHO conducted conflicts of interest at the planning stage for all PIs who submitted a written declaration that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
The NUTRIMAM Study Team:
Ishita Mostafa, Jafrin Ferdous, Irin Parvin, Hasan Hafizur Rahman, Kazi Nazmus Saqeeb, Mohammod Jobayer Chisti, Tahmeed Ahmed, Karim Manji, Christopher P. Duggan, Christopher Sudfeld, Sarah Somji, Rodrick Kisenge, Saidah Mohamed Bakar, Mohamed Bakari Kheri, Karen L. Kotloff, Samba O. Sow, Dilruba Nasrin, Milagritos Tapia, Adama M. Keita, Fadima C. Haidara, Jane Jauma, Farah Naz Qamar, Tahir Yusufzai, Sabeen Siddiqui, Naveed Ahmed, Haobijam Prabhabati Devi, Priyanka Mandal, Usha Dhingra, Srashti Sharma, Nav Ratan Kumar Gupta, Arup Dutta, Jitendra Kumar, Sunil Sazawal, Ayesha De Costa, Ameena Goga, Agnese Iuliano, Nigel Rollins, and Hiresh Tiwary
References
- 1.World Health Organization. Child mortality (under 5 years). 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/levels-and-trends-in-child-under-5-mortality-in-2020. Accessed 20 Oct 2023. [Google Scholar]
- 2.World Health Organization. Levels and trends in child malnutrition child malnutrition: UNICEF/WHO/World Bank Group Joint Child Malnutrition Estimates: Key findings of the 2023 edition. New York: World Health Organization; 2023.
- 3.Organization WH. WHO child growth standards and the identification of severe acute malnutrition in infants and children: joint statement by the World Health Organization and the United Nations Children’s Fund. 2009. [PubMed] [Google Scholar]
- 4.de Onis M, Borghi E, Arimond M, Webb P, Croft T, Saha K, et al. Prevalence thresholds for wasting, overweight and stunting in children under 5 years. Public Health Nutr. 2019;22(1):175–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang CY, Trehan I, Wang RJ, Thakwalakwa C, Maleta K, Deitchler M, Manary MJ. Children successfully treated for moderate acute malnutrition remain at risk for malnutrition and death in the subsequent year after recovery. J Nutr. 2013;143(2):215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Guideline: updates on the management of severe acute malnutrition in infants and children. Geneva: World Health Organization; 2013. [PubMed]
- 7.Food and Agriculture Organization, United Nations High Commissioner for Refugees, United Nations Children's Fund, World Food Programme, World Health Organization. Global action plan on child wasting: a framework for action to accelerate progress in preventing and managing child wasting and the achievement of the Sustainable Development Goals. 2020. Available from: https://cdn.who.int/media/docs/default-source/nutritionlibrary/publications/malnutrition/global-action-plan-child-wasting_58c36b82-d381-41c8-96f8-19332a8ea168.pdf?sfvrsn=79760143_3&download=true. Accessed 23 Oct 2023.
- 8.Das JK, Salam RA, Saeed M, Kazmi FA, Bhutta ZA. Effectiveness of interventions for managing acute malnutrition in children under five years of age in low-income and middle-income countries: a systematic review and meta-analysis. Nutrients. 2020;12(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teta I, Foudjo BUS, Nielsen JN, Oben J, Nguefack-Tsague G, Ntentie FR, et al. Outcomes of a food voucher program and factors associated with the recovery rate of children with moderate acute malnutrition in Far North Cameroon. J Health Popul Nutr. 2023;42(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen RY, Mostafa I, Hibberd MC, Das S, Mahfuz M, Naila NN, et al. A microbiota-directed food intervention for undernourished children. N Engl J Med. 2021;384(16):1517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somassè YE, Dramaix M, Bahwere P, Donnen P. Relapses from acute malnutrition and related factors in a community-based management programme in B urkina F aso. Matern Child Nutr. 2016;12(4):908–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhandari N, Mohan SB, Bose A, Iyengar SD, Taneja S, Mazumder S, et al. Efficacy of three feeding regimens for home-based management of children with uncomplicated severe acute malnutrition: a randomised trial in India. BMJ Glob Health. 2016;1(4):e000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abitew DB, Yalew AW, Bezabih AM, Bazzano AN. Predictors of relapse of acute malnutrition following exit from community-based management program in Amhara region, Northwest Ethiopia: an unmatched case-control study. PLoS ONE. 2020;15(4):e0231524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daures M, Phelan K, Issoufou M, Kouanda S, Sawadogo O, Issaley K, et al. New approach to simplifying and optimising acute malnutrition treatment in children aged 6–59 months: the OptiMA single-arm proof-of-concept trial in Burkina Faso. Br J Nutr. 2020;123(7):756–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen LC. Interactions of diarrhea and malnutrition: mechanisms and interventions. In: Lincoln C, Chen NSS, editors. Diarrhea and malnutrition: Interactions, mechanisms, and interventions. New York: Springer; 1983. p. 3–19. [Google Scholar]
- 16.Tomkins A, Watson F. Malnutrition and infection − a review − nutrition policy discussion paper No. 5. 1989. Available from: https://www.unscn.org/layout/modules/resources/files/Policy_paper_No_5.pdf. Accessed 01 Aug 2024.
- 17.Walson JL, Berkley JA. The impact of malnutrition on childhood infections. Curr Opin Infect Dis. 2018;31(3):231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerrant RL, Schorling JB, McAuliffe JF, De Souza MA. Diarrhea as a cause and an effect of malnutrition: diarrhea prevents catch-up growth and malnutrition increases diarrhea frequency and duration. Am J Trop Med Hyg. 1992;47(1 Pt 2):28–35. [DOI] [PubMed] [Google Scholar]
- 19.Hooli S, Colbourn T, Lufesi N, Costello A, Nambiar B, Thammasitboon S, et al. Predicting hospitalised paediatric pneumonia mortality risk: an external validation of RISC and mRISC, and local tool development (RISC-Malawi) from Malawi. PLoS ONE. 2016;11(12):e0168126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hooli S, Colbourn T, Lufesi N, Costello A, Nambiar B, Thammasitboon S, et al. Correction: predicting hospitalised paediatric pneumonia mortality risk: an external validation of RISC and mRISC, and local tool development (RISC-Malawi) from Malawi. PLoS ONE. 2018;13(2):e0193557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ngari MM, Fegan G, Mwangome MK, Ngama MJ, Mturi N, Scott JAG, et al. Mortality after inpatient treatment for severe pneumonia in children: a cohort study. Paediatr Perinat Epidemiol. 2017;31(3):233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black RE, Allen LH, Bhutta ZA, Caulfield LE, De Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. The lancet. 2008;371(9608):243–60. [DOI] [PubMed] [Google Scholar]
- 23.Chan A-W, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, SPIRIT, et al. explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;2013:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Food and Agriculture Organization, World Health Organization. Microbial safety of lipid-based ready-to-use foods for management of moderate acute malnutrition and severe acute malnutrition–second report. Rome: Food & Agriculture Org.; 2021.
- 25.United Nations Children's Fund. Product specification sheet: multiple micronutrient powder (MNP). UNICEF Supply Division; 2021. Available from: https://www.unicef.org/supply/media/16661/file/S1580201-Multiple-Micronutrient-powder-MNP-15-components-Specification.pdf. Accessed 20 Oct 2023.
- 26.World Health Organization Department of Child Adolescent Health. Handbook IMCI: integrated management of childhood illness. Geneva: World Health Organization; 2005.
- 27.World Health Organization. Training Course on Child Growth Assessment. Geneva: WHO; 2008. Available from: https://iris.who.int/bitstream/handle/10665/43601/9789241595070_A_eng.pdf?sequence=1. Accessed 20 Oct 2023. [Google Scholar]
- 28.Guideline IH. Integrated addendum to ICH E6 (R1): guideline for good clinical practice E6 (R2). Current Step. 2015;2:1–60. [Google Scholar]
- 29.World Health Organization. Essential nutrition actions: mainstreaming nutrition through the life-course. 2019. [Google Scholar]
- 30.World Health Organization. Supplementary foods for the management of moderate acute malnutrition in children aged 6–59 months: World Health Organization; 2023. Available from: https://www.who.int/tools/elena/interventions/food-children-mam#:~:text=In%20children%20aged%206%E2%80%9359%20months%2C%20moderate%20acute%20malnutrition%20is,and%20less%20than%20125%20mm. Accessed 03 May 2024.
- 31.De Costa A, Duggan CP. All moderately wasted children are at risk, but some are more at risk than others. Am J Clin Nutr. 2021;114(3):835–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Rationale behind targeted energy intake of 130 kcal/kg body weight per day.
Additional file 2. Food supplements composition.
Data Availability Statement
The datasets generated during the current study will be available from the corresponding author on reasonable request.