Figure 4. N619K mutation reduces Ca2+ binding at site-G, external Ca2+ block, and Ca2+ permeability.
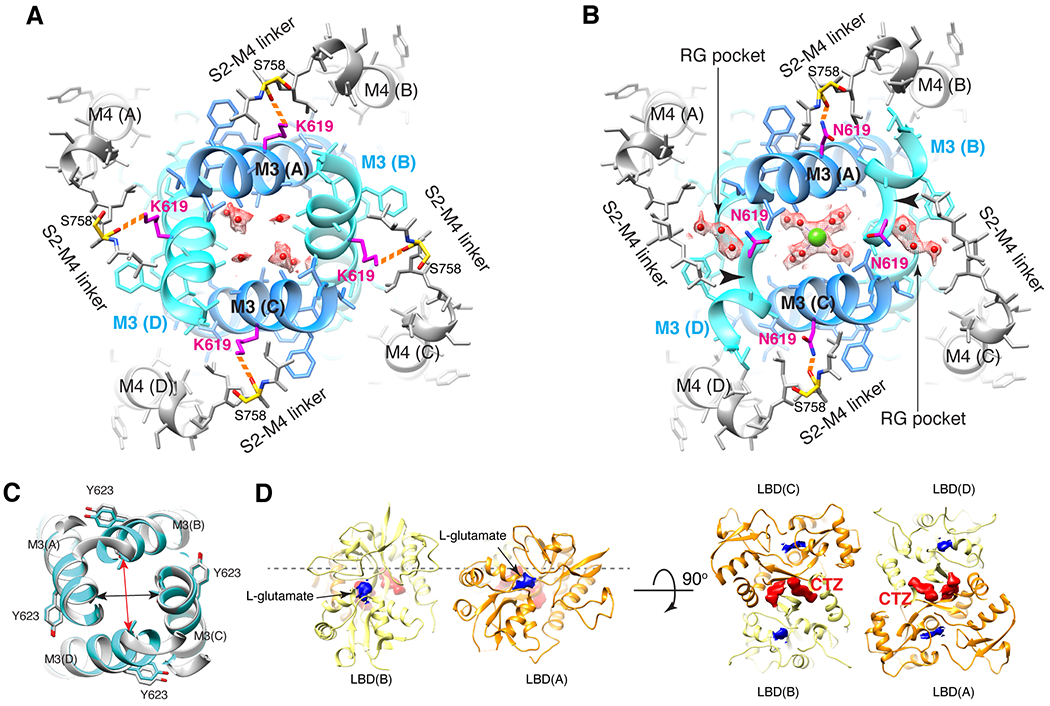
A-B. The site-G of the mutant A2iQ(N619K)/γ2(KKEE) (A) and WT A2iQ/γ2(KKEE) (B) viewed from the extracellular side. The M3 kink (arrowhead in B) is only present in WT. The central pore density is absent in the mutant, while the Ca2+ (green sphere) is found in the WT. Water in red sphere. Density map in mesh. The side chains of mutated K619 and WT counterpart N619 are in magenta. The S758 in yellow. The K619 interacts with the carbonyl oxygens of S758 (orange dashed lines). In WT the RG pocket filled with water occupies the space between N619 and S758 in the B/D subunits. In A/C subunits, the N619 interacts with the carbonyl oxygens of S758 (orange dashed lines). C. Overlay of site-G of WT (light gray) and N619K (cyan) receptors, aligned using the M3 segment residues 610-615. The pore is narrower in the B/D subunit axis (red arrow: distances of T617 Cα of B/D; 12.38Å in N619K vs. 13.57Å in WT) and wider in the A/C axis (black arrow: distances of T617 Cα of A/C; 11.84 in N619K vs. 11.56Å in WT). D. The ribbon model of LBD gating ring of LBDconf1 in A2iQ(N619K)/γ2(KKEE) complex (Supplementary Table 1) displayed at two orthogonal views. LBD monomers of A/C and B/D subunits in orange and light yellow, respectively. The densities for glutamate and CTZ are in blue and red, respectively. The conf2 is also fully liganded as in conf1 (not shown).
