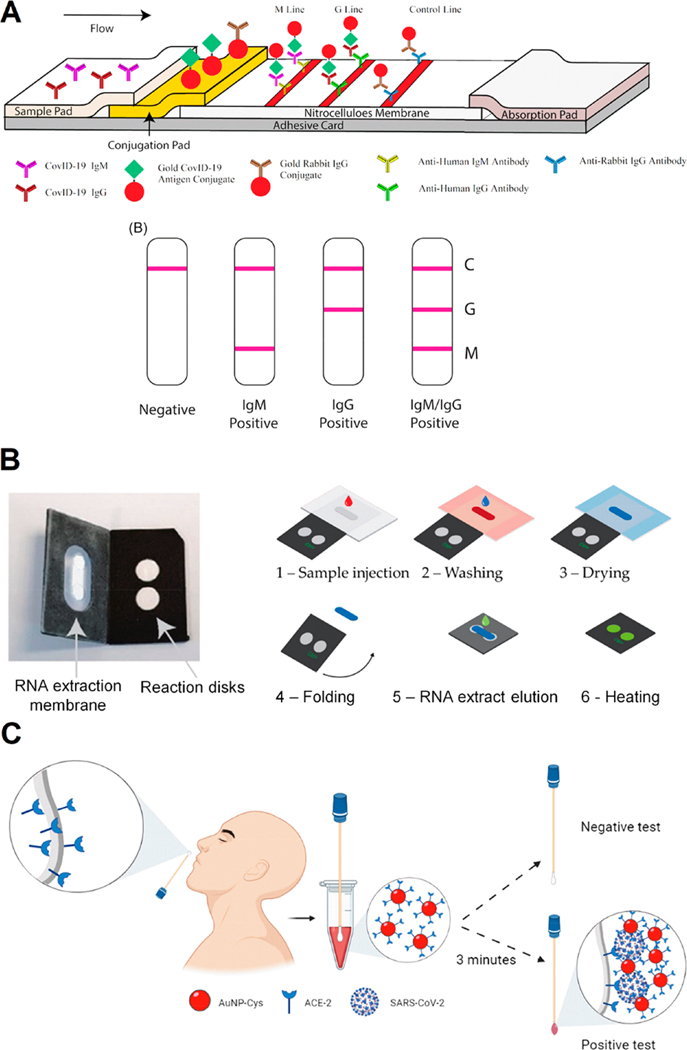
Figure 4.
Colorimetric approaches for COVID-19 diagnosis. (A) Schematic illustration of rapid SARS-CoV-2 IgM-IgG combined antibody test with an illustration of different testing results; C, means control line; G, means IgG line; M, means IgM line. Reproduced from ref 32. Copyright 2020 Li et al. Journal of Medical Virology Published by Wiley Periodicals, Inc. (B) POC LAMP-based device and workflow. (1) The sample (with lysed virus) is injected onto the extraction membrane. (2) Washing. (3) Drying of the extraction membrane. (4) Folding of the device so the extraction membrane comes into contact with the two reaction disks. The LAMP mix and primers (COVID-19 and human 18S RNA for the test and control disk, respectively) are freeze-dried on both disks, permitting reverse transcription and amplification. (5) Elution of the RNA from the capture membrane to the reaction disks, subsequently sealing with PCR tape. (6) Heating at 65 °C. Read-out in real-time with intercalating agent SYTO82. Adapted with permission under a Creative Commons CC BY 4.0 License from ref 113. Copyright 2021 Garneret et al. (C) Schematic representation of the use of cotton swab colorimetric biosensor using AuNPs functionalized with ACE2. The colored cotton swab indicates a positive result for SARS-CoV-2. Reproduced with permission from ref 30. Copyright 2021 ACS.