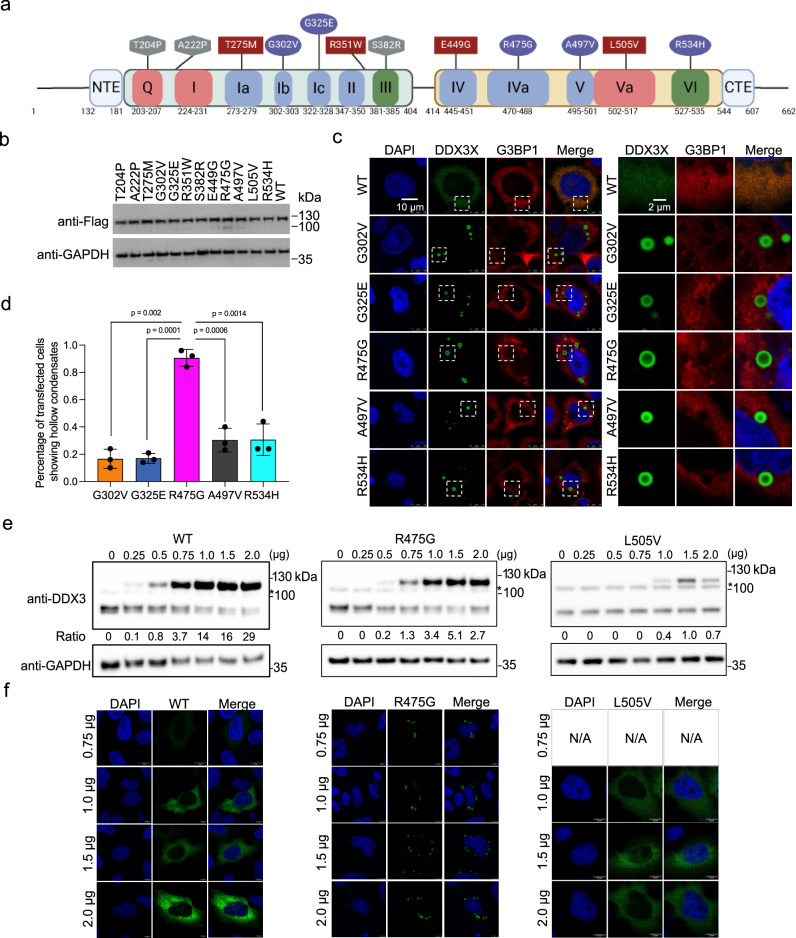
Fig. 1. A subset of DDX3X disease mutants form unique hollow condensates in cells.
a Schematic of the conserved domains and motifs of DDX3X and the locations of each disease variant used in this study. Motifs responsible for ATP binding and hydrolysis (Q, I, and Va) are in red, RNA binding (Ia, Ib, Ic, II, IV, IVa, V) in blue, and coordination of ATPase and RNA binding (III and VI) in green. Diffuse mutants are shown in squares, speckled mutants in hexagons, and hollow mutants in ovals. N-terminal IDR = a.a. 1–131, N-terminal extension (NTE) = a.a. 132–181, N-terminal RecA-like domain = a.a. 182–403, linker = a.a. 405–413, C-terminal RecA-like domain = a.a.414–543, C-terminal extension (CTE) = a.a. 545–607, C-terminal IDR = 608–662. Created in BioRender. Owens (2023) BioRender.com/k65s989. b Western blots showing that the mClover3-tagged DDX3X variants were expressed at similar levels in HeLa cells. c Representative images showing the localization of mClover3-tagged WT DDX3X or the indicated variants and mCherry-tagged G3BP1 in HeLa cells. The white boxes indicate regions zoomed on the right. Scale bars, 10 µm and 2 μm. d Quantified percentage of HeLa cells containing DDX3X variant hollow condensates. Values represent means ± s.d. from three independent measurements of at least 20 cells each. Significance was calculated using a two-tailed t-test e Representative Western blots showing expression of exogenous mClover3-tagged WT, R475G, and L505V DDX3X and endogenous WT DDX3X. * indicates a non-specific band produced by this antibody. Ratios of exogenous/endogenous band intensity included below each lane. Titrations were performed in triplicate, additional blots can be found in the Supplementary Fig. 1. f Immunofluorescence imaging showing cellular distribution patterns of WT, R475G, and L505V DDX3X at the indicated plasmid concentrations. Source data are provided as a Source Data file.