Abstract
Background: The Stroop task was used to investigate differences in cognitive function between Long COVID (LC), Myalgic Encephalomyelitis / Chronic Fatigue Syndrome (ME/CFS) and healthy control subjects. Methods: Subjects viewed four color words or neutral (XXXX) stimuli with the same (congruent) or different color ink (incongruent). Cognitive conflict was inferred from response times for pairings of prestimuli and subsequent stimuli. Overall effects were assessed by univariate analysis with time courses determined for binned response times. Results: LC and ME/CFS had significantly longer response times than controls indicating cognitive dysfunction. Initial response times were ranked LC > ME > HC, and decreased according to power functions. At the end of the task (900s), times were ranked LC = ME > HC. Response times were significantly slower for stimuli following an incongruent prestimulus. Time series for Stroop effect, facilitation, interference, surprise index and practice power law parameters were generally similar in LC, ME/CFS and HC suggesting comparable patterns for recruitment of cognitive resources. The prestimulus data were analyzed and generated positive Stroop and interference effects that were distinct from stimulus effects. Conclusion: LC and ME/CFS have global slowing of response times that cannot be overcome by practice suggesting impaired communications between network nodes during problem solving. Analysis of matched prestimulus – stimulus effects adds a new dimension for understanding cognitive conflict. Brief Summary: Cognitive dysfunction in Long COVID and ME/CFS was demonstrated using the Stroop task which found global slowing of response times and limitations of practice effects.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-75651-3.
Keywords: Stroop effect, Facilitation, Interference, Practice, Cognitive dysfunction, Long COVID, Myalgic encephalomyelitis / chronic fatigue syndrome, ME/CFS
Subject terms: Neuroscience, Cognitive neuroscience, Attention, Cognitive control, Problem solving
Introduction
Myalgic Encephalomyelitis / Chronic Fatigue Syndrome (ME/CFS) is a chronic disease with disability, fatigue, postexertional malaise (PEM), cognitive lapses, nonrefreshing sleep, interoceptive distress, pain and orthostatic complaints1–6. PEM is the key manifestation. Physical, cognitive or emotional exertion at greater than usual levels, lead to symptom relapse that may be of immediate onset or delayed by hours and that forces patients to remain in bed or house bound until recovery. There have been multiple epidemic outbreaks, but most cases are sporadic. Patients often recall a severe flu-like illness that never resolved. This symptom profile mirrors Long COVID (LC), the persistent fatiguing illness that does not abate after acute SARS-CoV2 infection6. Long-COVID (LC) participants were selected if they had one or more of these symptoms, moderate or worse severity, onset less than 3 months following COVID-19 infection and persistence for at least 3 months according to the WHO working case definition7. ME/CFS (ME) patients met International Case Criteria (ICC)3or 1994 Center for Disease Control (“Fukuda”) criteria2. It is not known if ME/CFS and Long COVID are parallel symptom complexes that share the same longitudinal brain, metabolic or immune pathologies8. There are no diagnostic tests or approved therapies for either disorder. Symptoms of “brain fog” and cognitive dysfunction are diagnostic features of both ME/CFS and LC9–14.
Neuropsychological testing in ME/CFS has quantified cognitive impairment affecting visuospatial immediate memory (Hedges’ g = 0.55, p = 0.007), reading speed (g = 0.82, p= 0.0001), processes of storage, retrieval and recognition that are inherent in episodic verbal memory, recovery of visual memories and low efficiency in attentional abilities9. Reaction times were impaired in simple stimulus response tests and more complicated choice tests15–27. Hedges’ g was moderately strong (0.66 and 0.50, respectively) but heterogeneity was high (Q = 54.1 and 17.36, respectively) in meta-analyses. Executive functioning does not appear to be affected.
More challenging tests such as the Stroop task28,29 measure a range of executive, salience and attention network functions. The test requires matching the color of ink for a written word in one panel with a color in a second panel. For example, matching the word RED written in red ink is the congruent condition (Fig. 1). The incongruent condition is when the word and its ink color do not match, such as the word RED in yellow ink. We included a neutral condition where XXXX is presented which is not a word but still requires analysis for colour conflict and motor response. In each case, the automatic response is to read the word. This is adequate in the congruent condition when the ink and word match. However, if the word and its color do not match (incongruent condition) then cognitive processes of word reading and color identification interfere with each other and generate a conflict that requires longer processing for conflict recognition, error checking and adaptation30. The conflict is between the competing demands of the task to name the color versus the interference from automatic word reading31. Word reading reflects the speed of visual search and language centers for word recognition. Color naming depends on working memory and speed of visual search. Color-word association relies on working memory, conflict monitoring, and speed of visual search31. The cognitive interference of word reading on colour identification29,32impacts ME/CFS more than controls (Hedges’ g 0.40 to 0.51)17,20,26,33–35. Processing speed in the color / word versions of the Stroop task was compromised in ME/CFS based on Hedges’ g of 0.76 to 0.82 in literature reviews18,23,35,36.
Figure 1.

Stroop task. Three examples of each of the three categories of the colour-word Stroop task stimulus: ‘C’ congruent: colour of upper word agrees with its meaning, ‘I’ incongruent: colour of upper word differs from its meaning, and ‘N’ neutral: the upper coloured line does not contain a word (XXXX). The task was to answer ‘Does the colour of the upper word agree with the meaning of the lower word?’ and press one of two buttons for “yes” or “no”. The correct response is indicated by Y or N at the right hand side of each example.
LC have slower response times (Rt) when performing the Stroop task37that may be related to dysfunctional attention with hypoarousal38and increased brainstem midbrain connectivity by fMRI39. We analyzed past data to verify that response times were significantly slower in ME/CFS and LC than HC.
The Stroop test has evolved from lists of words written on three hardcopy pages to be read as fast as possible to two words on a computer screen which require the subject to decide “Does the colour of the upper word agree with the meaning of the lower word?” and then press one of two buttons for yes or no29. A refinement of this task found that stimulus response times were dependent on the preceding stimulus (prestimulus)40as predicted by the congruency sequence or Gratton effect41. The effect of the preceding stimulus on the response time (prestimulus effect) has been linked to the anterior cingulate cortex42. We proposed that ME/CFS and LC subjects would have greater impairment caused by the incongruent presentations and exaggerated prestimulus effects compared to HC.
For clarity, we denote prestimulus in lower case (c, n, i) and subsequent Stimulus in Upper Case (C, N I). Pairings of prestimulus and Stimulus appear as prestimulus then Stimulus (e.g. cN). Responses times were reported for the Stimulus.
Repeated presentations lead to practice effects with progressively faster response times that decay according to power laws43. Practice may be a manifestation of cognitive automatization effects that may differ between ME/CFS, LC and HC as a result of disease mechanisms. Constants and exponents of power relationships were compared to investigate this hypothesis. Rt data were binned to quantify the serial effects44 and to assess differences between groups. We proposed that practice effects and power-function speed-up would be slower to develop in ME/CFS and LC compared to faster adaptation in HC.
The theory of automaticity provided a null hypothesis by proposing that improvement of Rt with practice would be due to the increase in knowledge base following repeated presentations rather than changes in cognitive strategies43. This hypothesis predicted that the Rt for each type of presentation and their standard deviations would decrease by the end of the task according to a power43or exponential45 function.
Cognitive processes utilized in the Stroop task have been quantified as the Stroop (congruency) Effect (Rt for Incongruent minus Congruent Stimulus), Facilitation (Congruent - Neutral) and Interference (Incongruent – Neutral)44,46,47. There is significant disagreement about the order and level of processing events during cognition and potential anatomical loci for each analytical process. We predicted these indices would be different between ME/CFS, LC and HC and show diverse patterns of change over time (practice effects). In addition, we adapted these indices to the prestimulus to study possible consequences of each type of presentation on Rt of the following consecutive stimulus. These attributes have not been studied in the context of cognitive dysfunction in ME/CFS or LC.
Results
Demographics
Two cohorts had fMRI scans with Stroop tasks in 2016 or 2023. Sex as a biological variable was assessed in the context of ME/CFS and its elevated ratio of females to males. Groups were equivalent for age and gender (Table 1).
Table 1.
Demographics.
| Cohort | 2016 | 2023 | ||||
|---|---|---|---|---|---|---|
| HC | ME ICC | ME Fukuda | HC | ME ICC | LC | |
| N | 27 | 18 | 24 | 16 | 31 | 19 |
| female/male | 19/8 | 12/6 | 20/4 | 11/5 | 24/7 | 14/5 |
| Age mean | 43.1 | 43.3 | 49.7 | 38.9 | 43.1 | 47.9 |
| Age SD | 13.7 | 10.7 | 11.5 | 12.6 | 10.9 | 13.3 |
Univariate analysis
Variation in response time (Rt) was investigated by univariate general linear model for simple main fixed effects with Sidak correction for multiple comparisons. Rt was the dependent variable with independent variables (fixed factors) of group (HC, LC, ME), prestimulus (n, c, i), and Stimulus (N, C, I) and elapsed time as a covariate.
The theory of automaticity predicted that the mean Rt and SD would decrease as the number of presentations increased43. This was examined in the univariate model by assessing the change in variance with time using the Modified Breusch-Pagan Test for Heteroskedasticity. Homoskedasticity is defined when the variance (SD) does not depend on the magnitude of the variable (Rt), while heteroskedasticity indicates variances grow larger as values become higher. The homoskedasticity hypothesis was falsified (Chi-Square 206.3, 1 degree of freedom, p = 10–258) indicating that variances were larger at higher values of Rt. Because Rt was highest at the start of the task and decreased progressively, we can assume that the variance of Rt decreased from start to end of the task. This result was consistent with the automaticity theory and concept that task performance improves with the continued accrual of additional practice43. The decrease in Rt with time indicated a significant practice effect over the duration of the Stroop task across all groups suggesting that the practice effect is inherently heteroskedastic. Elapsed time had the largest impact on the changes in variance (p = 1.6 × 10-59) and accounted for the heteroskedacity (SOM Table S1). Disease (p = 0.00059) and prestimulus (p = 0.0021) had smaller impacts. Therefore, the Rt data were binned by time to study the time course of practice for each disease group, prestimulus and Stimulus presentation type (see below).
Estimated marginal means for Rt ranked the groups as LC (1.68s [1.65 to 1.71] mean [95%CI]) > ME (1.60s [1.58 to 1.61], p = 6.9 × 10-7) > HC (1.37s [1.35 to 1.39], p = 10–258 vs. both LC and ME) (SOM Table S2). This suggested global slowing of neural communication during the task in LC and ME.
The prestimuli were ranked incongruent (i, 1.73s [1.71 to 1.75]) > congruent (c, 1.50s [1.48 to 1.53], p = 10–258 vs. incongruent) > neutral (n, 1.41s [1.39 to 1.43], p = 10–258 vs. incongruent and 4.7 × 10-9 vs. congruent) (SOM Table S3). The group x prestimulus cross-product had the same relationships for all three groups with the three prestimuli ranked i > c > n and LC > ME > HC (SOM Table S4). Subtraction of Rt values of prestimuli found similar Stroop effects (RT i – Rt c), facilitation (Rt n – Rt c) and interference (Rt i – Rt n) in the three groups based on the univariate data.
The overall Rt for Stimuli were closely bunched but were ranked Congruent (1.60s [1.58 to 1.62]) > Incongruent (1.54s [1.52 to 1.56] p = 0.00088) = Neutral (1.50s [1.48 to 1.53] p = 1.8 × 10-18) (SOM Table S5). Incongruent and Neutral Rt were equivalent (p = 0.053).
The group x Stimulus cross-product ranked Rt of Congruent and Incongruent Stimuli as LC > ME > HC. The Stroop effect and facilitation were negative while interference was positive for all three groups (SOM Table S6)47.
Estimated marginal means for the prestimulus x Stimulus cross-product emphasized the predominant effect of the incongruent prestimulus to prolong Rt compared to congruent and neutral for each combination of Stimulus. In general, the incongruent prestimulus caused the highest Rt values without regard for the Stimulus (SOM Table S7). The incongruent prestimulus effect was found for each group suggesting the same hierarchy of cognitive difficulty and relative effort. However, the longer Rt values demonstrated the significant cognitive problems in ME and LC groups.
The group x prestimulus x Stimulus triple cross product highlighted the overall dominant effect of the incongruent prestimulus within HC, LC and ME groups (Fig. 2, SOM Tables S8 and S9). LC and ME had significantly longer Rt for all pairings compared to HC signifying cognitive dysfunction. LC had longer Rt than ME for iC and nC pairings where different prestimuli preceded the Congruent Stimulus. This suggested difficulties for LC to process the Congruent Stimulus.
Figure 2.
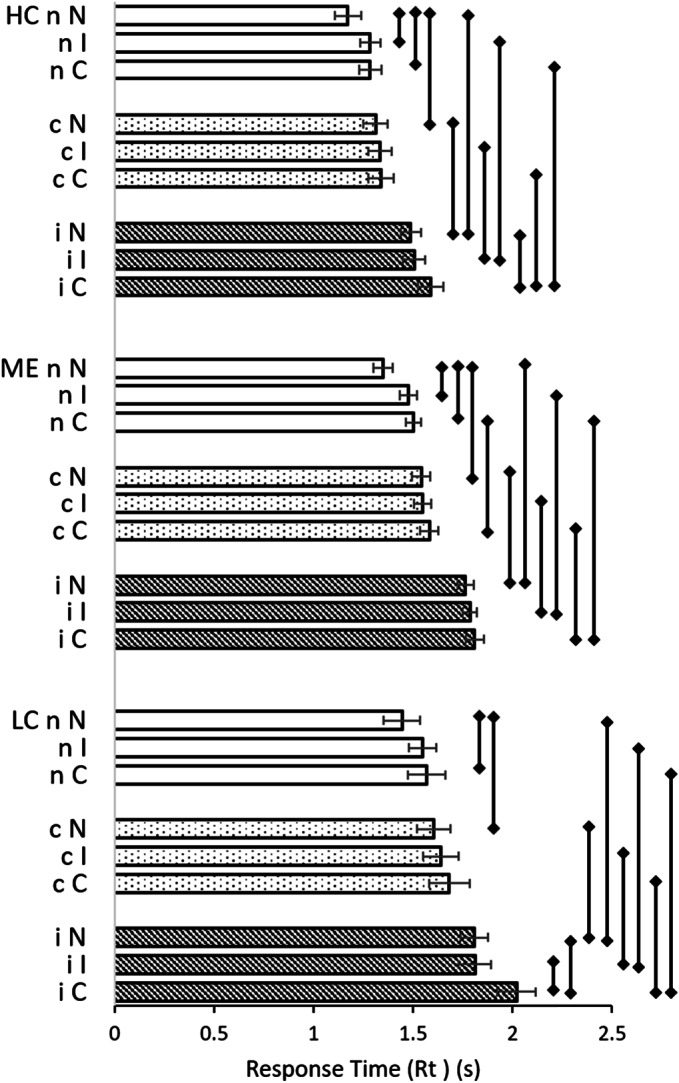
Rt for each pairing and group. Univariate analysis generated mean Rt with 95%CI error bars for neutral (white bars), congruent (dotted bars) and incongruent (diagonal lines) prestimuli in HC, ME and LC groups. Significant differences in Rt within each group were indicated by error lines to the right of data bars (p < 0.05). The predominant significant effect of the incongruent prestimulus was evident. LC and ME had significantly longer Rt than HC for each pairing (not indicated). LC was prolonged compared to ME for iC and nC. Data are in SOM Tables S8 and S9.
Power analysis of Rt time courses for practice effects
Rt time courses were fitted to power curves (Rt = constant x 10exponent) where the constant was an approximation of the Rt y-intercept at the start of the task and the exponent was a measure of the change in Rt over time that has been likened to the rate of learning43. Exponential decay was another alternative45 but power curves accounted for higher explained variance here. It was noted that the constant and exponent were highly correlated (R2 = 0.80) across the entire dataset suggesting that the highest initial Rt values had the fastest rate of decrease. Based on the univariate findings, we predicted that the initial Rt (constant) and rate of learning (exponent*(-1)) would be higher in LC and ME than HC and that the practice effects would be larger in LC and ME so that all groups had the same Rt at the end of the task (Rt extrapolated to 900s). Parameters were calculated for the nine pairings then compared between groups by paired t-tests.
As predicted, the constant and exponent*(-1) were highest in LC, but values were ranked LC > ME = HC (Table 2). Therefore, LC had the greatest cognitive deficit at the start of the task and had the fastest rate of learning. ME was not different from HC. However, Rt extrapolated to the end of the task (900s) were equivalent between LC and ME and higher than HC indicating that the disease groups had global cognitive deficits that could not be corrected by practice. The incremental change between 0 and 900 s (∆Rt) was larger for LC than ME. These practice effects may be a way to distinguish LC from ME although they may also indicate shorter vs longer duration of disease, respectively.
Table 2.
Power functions for practice effects. The overall average, SD and significant differences by paired t-test were calculated for power curve parameters determined from the nine prestimulus stimulus pairings (uncorrected p-values) (SOM Table S10).
| exponent x (-1) | constant (Rt at 0s) | Rt at 900s | ∆Rt | |
|---|---|---|---|---|
| HC | 0.078 ± 0.047 | 2.07 ± 0.53 | 1.19 ± 0.14 | 0.88 ± 0.61 |
| LC | 0.133 ± 0.050 | 3.57 ± 1.38 | 1.38 ± 0.14 | 2.19 ± 1.38 |
| ME | 0.069 ± 0.039 | 2.21 ± 0.46 | 1.36 ± 0.14 | 0.85 ± 0.50 |
| LC > ME | p = 0.00020 | p = 0.0051 | p = 0.22 | p = 0.0060 |
| LC > HC | p = 0.010 | p = 0.0082 | p = 4.8 × 10-6 | p = 0.018 |
| ME > HC | p = 0.33 | p = 0.33 | p = 9.4 × 10-7 | p = 0.82 |
Binned data for time course of practice effects
Table 2and the heteroskedacity outcomes assume that practice effects were constant for all prestimulus Stimulus pairings over the 900s time course. However, it was possible that time courses were different for congruent, incongruent or neutral presentations in the three disease groups. Other possible variations could be due to slower onset of practice effects or development of fatigue with slower Rt at the end of the task. Therefore, practice effects were evaluated further by examining the time course of changes in Rt using six bins with approximately equal numbers of stimulus presentations44. Bin 1 lasted 162s (21.5 stimuli) and the remainder ended at 294s (39.2 stimuli), 427s (56.9 stimuli), 563s (75.0 stimuli), 694s (92.5 stimuli) and 900s (120 stimuli). The average Rt for Congruent, Incongruent and Neutral Stimuli and congruent, incongruent and neutral prestimuli were determined for each binned group. Stroop Effect, Facilitation and Interference and their standard deviations, SEM and unpaired Student’s t-tests for differences from zero were calculated by propagation of errors48.
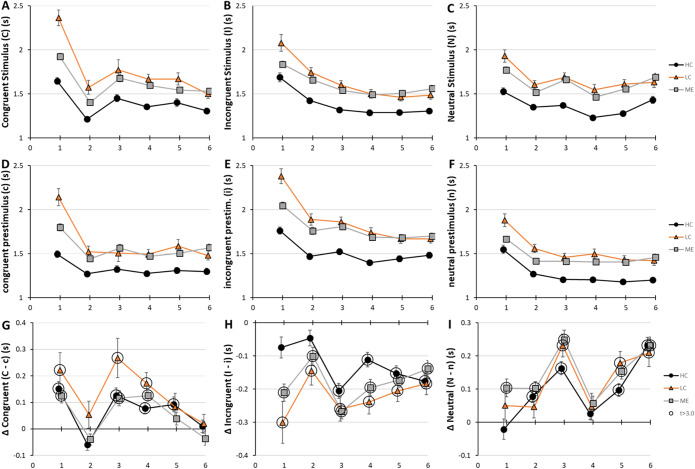
As seen in the univariate analysis for the complete 900s time course (Fig. 2), LC and ME had longer Rt than HC for stimuli and prestimuli in all bins (Fig. 3), again indicating the cognitive dysfunction in LC and ME. The first bin had the longest Rt values. Rt decreased progressively throughout the task confirming the power law practice effect. The decay curve for the Incongruent Stimulus was the smoothest and continued through bin 6 suggesting subjects may have focused on the Incongruent Stimulus throughout the task. Power function decay curves were seen for the incongruent and neutral prestimuli as well. In contrast, the congruent prestimulus showed a steep decrease from bin 1 followed by a flat curve that may suggest a rapid adaptation. The bin-to-bin changes in Rt were smooth with the exception of the Congruent Stimulus in bin 2 where Rt showed a large drop from bin 1 followed by an increase in bin 3. This dip was seen for all groups and may indicate transiently faster recognition of the Congruent Stimulus during the interval of bin 2 or shifting cognitive strategies throughout the task. The Neutral Stimulus had a small increase in Rt in bin 3 for LC and ME.
Figure 3.
Time course of practice effects. Rt (s) in each bin was plotted for HC (black circles and line), LC (orange triangles and line) and ME (grey squares and line) for each Stimulus (A-C), prestimulus (D-F) and differences (∆) in Rt between Stimulus and prestimulus presentations for Congruent, Incongruent and Neutral stimuli (G-I). Error bars indicate SEM. Large circles indicated p < 0.05, t > 3.0 for > 200 degrees of freedom. Data are in SOM Table S11.
Prestimulus and stimulus effects
The relative impact of prestimulus and Stimulus were seen by the delta (∆) for each matched pairing (Fig. 3G-I). We hypothesized that Rt would be longer for Stimulus than prestimulus and that ∆ values would all be positive and significantly greater than zero. However, this was not the case, in particular for ∆ = Incongruent Stimulus minus incongruent prestimulus (Fig. 3H). The overall patterns were highly similar between HC, LC and ME suggesting similar evolution of cognitive strategies for completing the Stroop task. The ∆ for Congruent Stimulus minus congruent prestimulus was significantly greater than zero for all three groups. However, ∆Congruent temporarily dropped to zero in bin 2 because of the faster response times to the Congruent Stimulus and in bin 6 because of the improving Rt for Congruent Stimulus with steady Rt for the prestimulus (Fig. 3G). The ∆ for Incongruent minus incongruent was negative in all bins indicating that the incongruent prestimulus induced significantly prolonged cognitive consequences that slowed down the Rt of the following Stimulus. ∆Incongruent had larger (negative) magnitudes for LC and ME than HC in bin 1, but values became equivalent between groups after bin 2. ∆Neutral started near zero but increased by bin 6 with the largest value corresponding to the small increase in Rt in bin 3 (Fig. 3C).
Stroop effects
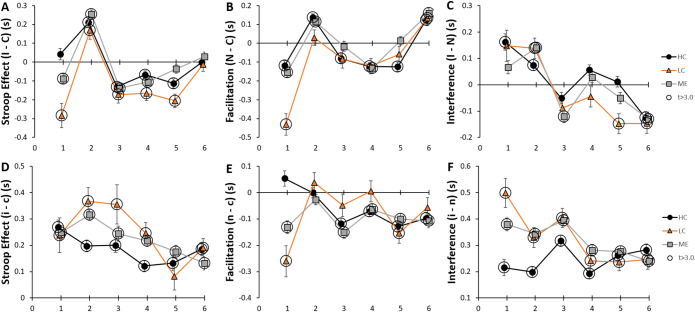
Surprisingly, Stroop effects (Incongruent – Congruent) for the Stimulus were found only in bin 2 for all three groups (Fig. 4A). Significant reverse Stroop effects were found in bin 1 for ME and LC and for all three groups in bins 3, 4 and 5. There was no net effect for any group in bin 6. This pattern was due to the exponential decay of Rt for Incongruent Stimulus (Fig. 3B) and shortening of Rt for the Congruent Stimulus in bin 2 (Fig. 3A). Stroop Facilitation (Neutral – Congruent) followed a similar trend with negative values in bin 1 that increased in bin 2 (Fig. 4B). Interference (Incongruent – Neutral) was significant in bins 1 and 2 but decreased in approximately linear fashion towards negative values in bin 6 for all three groups (Fig. 4C).
Figure 4.
Time course for Stroop effects of prestimulus and Stimulus. Stroop, Facilitation and Interference effects were shown for Stimulus (upper row, A-C) and prestimulus (lower row, D-F) in HC (black circles and line), LC (orange triangles and line) and ME (grey squares and line) were indicated by positive values that were significantly different from zero (large circles, p < 0.05, t > 3.0 for > 200 degrees of freedom). Error bars indicate SEM. Data are in SOM Table S12.
Because of the large effect of the incongruent prestimulus (Fig. 3E), Stroop effects were evaluated for each prestimulus (Fig. 4). Unlike the Congruent Stimulus, the congruent prestimulus caused positive Stroop effects with LC and ME groups having larger effects in bins 2 and 3 compared to HC (Fig. 4D). In the second half, Stroop effects became equivalent. Significant facilitation was present in the first bin for LC and ME (Fig. 4E) with values becoming equivalent between groups in the second half of the task. Stroop interference from the prestimulus was significantly higher for LC and ME than HC in bins 1 and 2 (Fig. 4F) before curves for the three groups converged together in the second half.
These curves demonstrate dynamic changes in Stroop effects over the course of the task with disease related reverse Stroop effects and facilitation for Stimulus in bin 1 for LC (Fig. 4A). The prestimulus had significantly larger Stroop, facilitation and interference effects in LC and ME (Fig. 4E, F) during the first half of the task.
Surprise
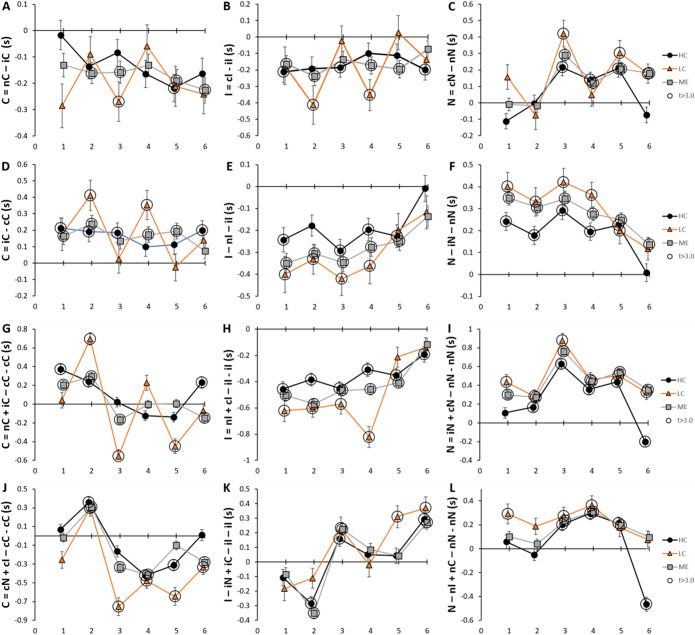
Presentation of pairings with different prestimuli and Stimuli such as cI, iC and iN may be considered a cognitive surprise and induce strategic reorientation. A strategic surprise index with three terms was adapted from Rt formulas of Carter et al42. (equations in SOM Table S13). The “surprise” term calculated the change in Rt when a prestimulus was followed by a dissimilar Stimulus in contrast to presentation of the same Stimulus twice (i.e. iC – iI). The next term corrected for “conflicts” with different prestimulus and stimulus (i.e. the average of cI and iC). The third term corrected for “same” sequential pairings (i.e. average of cC and iI). The index was calculated as “surprise” plus “same” minus “conflicts” and was interpreted to indicate strategic effects when it was positive and significant (sum > 0 and p< 0.05). The relevance is that significant positive results were correlated with anterior cingulate cortex activation for error monitoring42. We found values for the surprise term ranged from − 1 to + 1 for the six pairs of dissimilar Stimuli. Conflict terms ranged from 1.1 to 2.9 and the same term from 1.6 to 2.2.
All three groups had significant surprise indices for C in iC-cC (Fig. 5D) and N in cN-nN (Fig. 5C) and iN-nN (Fig. 5F). Looking closely at the iC and iN pairings suggested that when an I was presented and then became the prestimulus, there was a greater expectation that the next stimulus would be an I to give iI so that presentation of other stimuli (iC, iN) was viewed with “surprise” that required a greater or different cognitive response (strategic response) than would have occurred for iI. Surprise was not found for cI or nI suggesting a focus or vigilance for the appearance of I as the next Stimulus (Fig. 5B). The n prestimulus was not associated with surprise when different stimuli were presented next (nC, nI) (Fig. 5A and L). Other Stimuli gave negative and mixed responses that were more difficult to interpret (Fig. 5E, G-K).
Figure 5.
Surprise index. The time course for change in surprise indices was depicted for six pairings of Stimulus (upper two rows, A-F). Sums of these pairings gave the overall effects for Stimulus (third row, G-I) and prestimulus (bottom row, J-L). Data were shown for HC (black circles and line), LC (orange triangles and line) and ME (grey squares and line). “Surprise” was induced when the prestimulus and Stimulus did not match and the index was significantly greater than zero. Large circles indicate significant differences from zero (p < 0.05, t > 3.0 for > 200 degrees of freedom). Error bars indicate SEM. Data in SOM Table S14.
The impact of the Congruent Stimulus was defined by nC + iC-cC-cC (Fig. 5G) and represented the difference between dissimilar prestimuli (nC and iC) and the cC pairing. When the index was > 0 with p < 0.05, then the “surprise” of viewing C after n or i prestimuli was greater than the “nonsurprising” view of consecutive C stimuli (cC). The surprise index for bin 2 was significantly positive for all groups due to prolonged Rt when viewing nC and iC, and possible strategic consequences during this short window. The index was particularly elevated in bin 2 for LC. These results are likely to be related to the very short Rt for the Congruent Stimulus in bin 2 (Fig. 3) that predicts a relative shortening of the cC terms in the equation. Subsequently, the index moved to zero (not significant) indicating Rt became comparable for nC, iC and cC and that transiently engaged additional cognitive resources were no longer required or that a strategic plan was emplaced to deal with the C Stimulus during the latter half of the task. Bin 2 may expose a transient state during the evolution of problem solving with this Stroop task. This interpretation was supported by the effect of the congruent prestimulus (cN + cI-cC-cC) (Fig. 5J) where significant surprise effects for all three groups were found only during bin 2.
The index for the Incongruent Stimulus (cI + nI-iI-iI) did not elicit surprise (Fig. 5H) suggesting a concerted focus and potentially a strategic plan for anticipating and responding to future I Stimuli without regard for the preceding prestimulus. This was an unexpected result because we had anticipated that the I Stimulus would require additional resources for cognitive surveillance and word color conflict resolution to account for its prolonged Rt values. However, Rt for nI and cI were balanced by the iI terms in the formula suggesting that the Incongruent Stimulus itself was a persistent challenge without regard to its prestimulus. The generally linear upward trend may reflect significant practice effects for I (Fig. 3B).
iN + iC-iI-iI estimated the surprise and strategic effect of the incongruent prestimulus (Fig. 5K). During the first third of the task, the effects of dissimilar “surprises” (iN, iC) following the incongruent prestimulus were overcome by the longer Rt associated with the cognitively challenging but “unsurprising” repetition of I (iI). Note that this relationship must have changed over the course of the task because iC had longer Rt than iI (Fig. 2). The dynamic change was indicated by the general upward trend for the index and its positive values in the second half of the task. The strong practice effect for I and continuing predominance of the i prestimulus effect (Fig. 2) was the most likely explanation because the Rt for iI decreased more rapidly than iC (Fig. 5D) and iN (Fig. 5F). The I practice and i prestimulus effects were present in all three groups and so did not appear to be influenced by disease pathologies.
Index scores for the Neutral Stimulus were positive (iN + cN-nN-nN) (Fig. 5I) because of the overall ranking of prestimulus effects as i > c > n so that Rt for nN was faster (smaller) than iN and cN (Fig. 2). The index for neutral prestimulus (nI + nC-nN-nN) had a similar trend (Fig. 5L) because of the overall ranking of C > I = N for Stimulus Rt. HC bin 6 was an exception as both indices became negative because of a small uptick in Rt for N (Fig. 3C) that may suggest a relative loss of focus for this Stimulus or the onset of fatigue in HC.
Practice effects can follow power43or exponential45functions. Therefore, the Rt data were log transformed and used as the dependent variable in a simple main fixed effects univariate regression model with dependent variables of group, prestimulus, Stimulus, six bins with log of elapsed time as a covariate and Sidak correction for multiple comparisons. Data were contrasted for each prestimulus Stimulus pairing and LC, ME and HC groups. The time course in SD was plotted to determine if SD decreased with practice43. The log log transformations generated the highest R2 values.
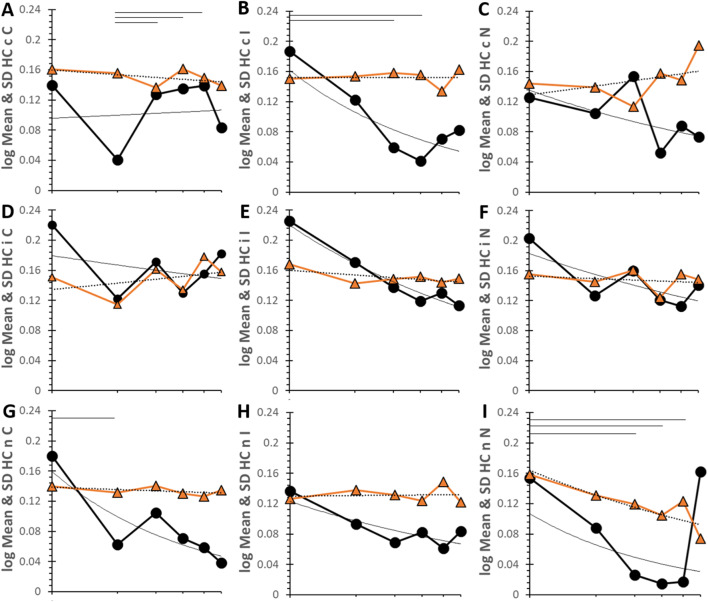
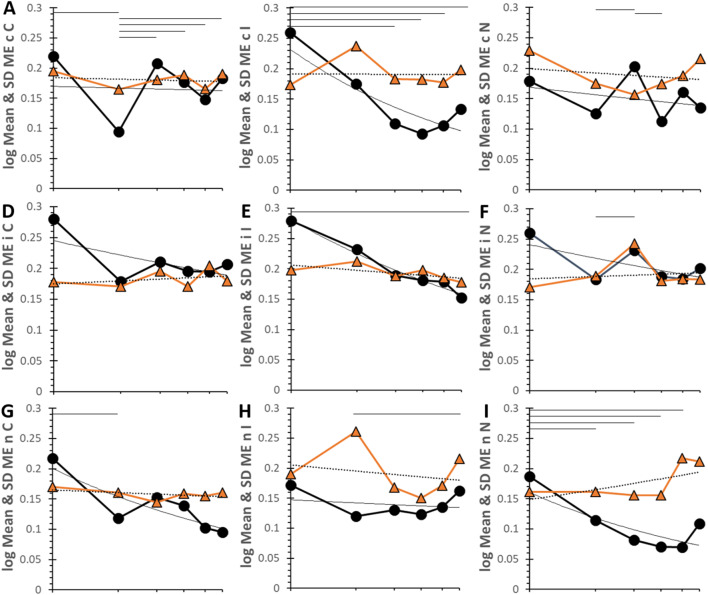
Geometric means for HC decreased significantly for iI (R² = 0.946), nC (R² = 0.712), nI (R² = 0.661), cI (R² = 0.568) and iN (R² = 0.523) (Fig. 6). Bin 1 had the highest values for cI, nC and nN. Exceptions with more horizontal curves were cC that had the significant drop in Rt in bin 2 as seen previously in Fig. 3, small bump for cN in bin 3 (R² = 0.328), flat curve for iC (R² = 0.0978), and large but nonsignificant increase of Rt for nN in bin 6 (R² = 0.180). Curves for SD were generally horizontal except for nN (R² = 0.681) and iI (R² = 0.421) that were downsloping.
Figure 6.
Log log time series for HC. Time courses are plotted for each pairing with log of mean and SD on the y-axis and log of bin on the x-axis. The top of the y-axis was 0.24s for HC. The six bins corresponded to 162s (21.5 stimuli), 294s (39.2 stimuli), 427s (56.9 stimuli), 563s (75.0 stimuli), 694s (92.5 stimuli) and 900s (120 stimuli). The mean (black circles, black line), SD (orange triangles and line) and power regression curves for mean (black line) and SD (dashed black line) were shown. Lines at the top of each graph indicate significant differences between means for time points (p < 0.05) in the univariate model. Data are in SOM Table S15.
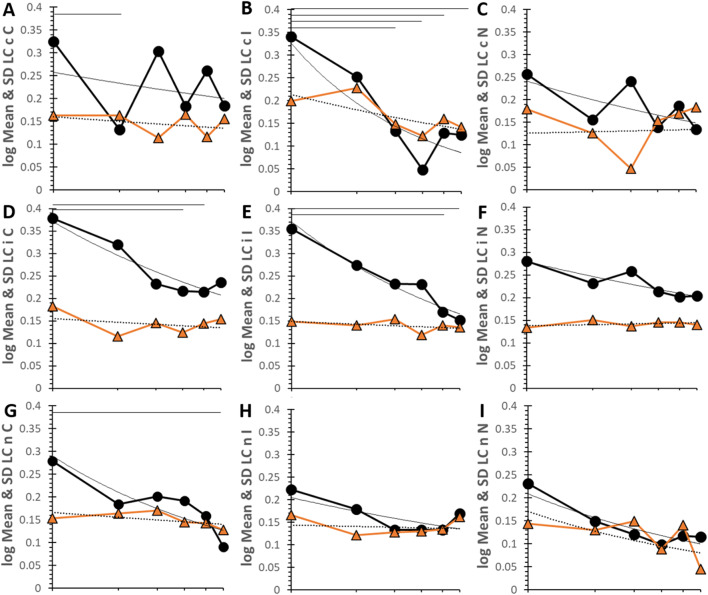
LC had higher geometric means of Rt as shown by the upper end of the y-axis at 0.4s compared to 0.24s for HC and 0.3s for ME (Fig. 7). cC had a sawtooth pattern with significant acceleration of mean Rt in bin 2 as seen previously. Bin 1 had the significantly slowest Rt but improved with practice for cC, cI, iC, iI and nC. Practice curves were significant for iI (R² = 0.923), iC (R² = 0.842), nN (R² = 0.815), iN (R² = 0.776), nC (R² = 0.680), nI (R² = 0.539) and cI (R² = 0.540). Log log curves for SD were horizontal except for cI (R² = 0.508). Although LC had large incremental improvements in Rt indicating significant practice effects (Table 2), LC was still significantly slower than HC in bin 6 for cC, iN and nI.
Figure 7.
Log log time series for LC. Time courses by six bins for LC. Time courses are plotted for each pairing with log of mean and SD on the y-axis and log of bin on the x-axis. The top of the y-axis was 0.4s for LC. The six bins corresponded to 162s (21.5 stimuli), 294s (39.2 stimuli), 427s (56.9 stimuli), 563s (75.0 stimuli), 694s (92.5 stimuli) and 900s (120 stimuli). The mean (black circles, black line), SD (orange triangles and line) and power regression curves for mean (black line) and SD (dashed black line) were shown. Lines at the top of each graph indicate significant differences between time points (p < 0.05) in the univariate model. Data are in SOM Table S15.
ME had intermediate Rt values (top of y-axis 0.3) (Fig. 8). Significant practice effects were found for iI (R² = 0.965), cI (R² = 0.699), nC (R² = 0.708), and nN (R² = 0.606). Rt for cI and nN were slowest in bin 1 but became significantly faster in later bins. Again, cC had its shortest Rt in bin 2. cN had a significant slowing of Rt in bin 3 compared to bins 2 and 4. The later time points in cI, nI and nN trended upwards suggesting possible fatigue effects or loss of attention. ME was significantly slower than HC in bin 6 for cC, iI, iN, nC and nI. Log log curves for SD were generally horizontal but had wider ranges for Rt and larger variations between bins than other groups. The SD curve for nN increased in bins 5 and 6 which was unlike the other pairings, LC and HC groups and may have indicated a decrease in attention or erratic Rt as a result of cognitive fatigue in ME.
Figure 8.
Log log time series for ME. Time courses by six bins for ME. Time courses are plotted for each pairing with log of mean and SD on the y-axis and log of bin on the x-axis. The top of the y-axis was 0.3s for ME. The six bins corresponded to 162s (21.5 stimuli), 294s (39.2 stimuli), 427s (56.9 stimuli), 563s (75.0 stimuli), 694s (92.5 stimuli) and 900s (120 stimuli). The mean (black circles, black line), SD (orange triangles and line) and power regression curves for mean (black line) and SD (dashed black line) were shown. Lines at the top of each graph indicate significant differences between time points (p < 0.05) in the univariate model. Data are in SOM Table S15.
Discussion
Performance in the Stroop task reflects informational conflict between the incongruent word meaning and colour and task conflict between relevant colour naming and irrelevant but automatic word reading49. The time courses shed light on how individuals dynamically adjust cognitive processes to optimise performance in the face of conflicting information. This insight contributes to our broader understanding of cognitive flexibility, which is crucial for adapting to changing environments and situations. The effects of prestimulus, Stimulus and task duration on response times (Rt) were determined from binned, univariate and logarithmically transformed analyses.
Overall, LC and ME/CFS had significantly prolonged Rt compared to HC confirming cognitive dysfunction.
The effect of Stimulus was ranked C > I = N. All three groups showed the large dip in Rt for C in bin 2 suggesting a common strategy for accelerated processing of C during this individual time period (Figs. 3, 6, 7 and 8). The smooth decrease in Rt for I throughout the task was parallel for the three groups indicating practice effects. Stroop effects were minimal for Stimulus (Fig. 4) possibly because of the heterogenous mix of congruent, incongruent and neutral stimuli that prevented proportion contingency effects that are more evident in tasks with predominantly congruent or incongruent presentations50and our use of the manual button box reporting system as opposed to verbal responses30.
The effect of the incongruent prestimulus to prolong the response time of the subsequent stimulus was a significant finding that was consistent with the congruency sequence or Gratton effect40–42. Further investigations found significantly different Stroop, facilitation and interference effects in LC and ME/CFS compared to HC in the first half of the task (Fig. 4). The effects became equivalent between groups in the second half. This dynamic evolution suggests that the incongruent prestimulus had a large impact on the cognitive processing and network interactions in LC and ME/CFS at baseline, and that practice effects sped up their Rts although they remained significantly slower than HC (Fig. 3).
From a pathophysiological perspective, ME and LC groups have significantly slower Rt to all stimuli compared to HC. This confirms earlier suppositions but extends those studies by investigating possible quantitative disease related changes in cognitive functions such as Stroop Effects, Facilitation, Interference, surprise and practice effects that can be defined by differential response times to Incongruent, Congruent and Neutral Stimuli. Univariate analysis ranked Rt for Stimulus as LC > ME > HC but the time course showed that practice effects evolved to rank Rt as LC = ME > HC at the end of the task. This confirmed the persistent residual cognitive deficit of LC and ME that improved but could not be overcome by practice. Rt improved most in the first half of the task suggesting this was the period of maximum adaptation. Rt was relatively constant in the second half suggesting a period of stabilization. Rt did not slow down again which would have suggested slower processing and development of cognitive fatigue. Comparison of the two halves by fMRI may provide insights into differences in mechanisms of cognitive processing during these periods and compensatory recruitment or network utilization between disease groups. Plausible molecular mechanisms to explain the prolonged Rt include defective neurotransmission related to reduced TPRM3 activity as shown for NK cells in ME and LC51, and loss of white matter integrity with increased axial diffusivity and potentially slowing of axon conduction times through long white matter tracts that connect regions required to complete the Stroop task52,53.
Practice improves performance for both colour recognition and word suppression dimensions43. Subjects were naïve to the Stroop task when recruited. They were given instructions on how to complete each test, but were not given trial runs to become familiar with the task. This prevented the development of learning strategies before starting the task and instead revealed their initial deficit (y-intercept of power curves) and progress with practice. Stimuli were viewed in random order making it more difficult to develop anticipatory strategies, and so were more likely to reveal conflict adaptation or practice effects.
Practice effects were examined using logarithmic time series (Figs. 6, 7 and 8). All three groups shared the significant transient acceleration of Rt in bin 2 with cC and slowing of cN in bin 3 that suggests common evolution of cognitive processing during the development of the most efficient network strategy. LC had the longest initial Rt values by power function analysis, average of bins and univariate analyses. ME was intermediate compared to HC. At the end of the task LC had the largest incremental improvements in Rt. Rt values were equivalent for LC and ME at the end of the task but were still slower than HC demonstrating their continuing cognitive deficits. The improvement in LC was reflected by the number of pairings that had significant practice effects (R2 > 0.5, effect size > 0.7) with LC having 7, HC having 5 and ME only 4. All three groups were significant for iI, nC and cI, with nI and iN also shared between LC and HC. Only LC was significant for nI, iN and iC. These data suggest LC began with the largest cognitive deficit (slowest initial Rt) and had the capacity to significantly improve their processing times by the end of the task. In contrast, ME had an intermediate level of deficit at the start but fewer significant practice effects and smaller incremental improvements than LC. This suggests that LC and ME may begin with differences in cognitive function and brain network activation in the first half of the task, but may evolve towards more similar network connections that are different from HC by the end of the task. The initial response in ME may indicate long term cognitive compensation given their longer duration of disease compared to the shorter subacute duration of LC. Differences in network connectivity between LC and ME can be tested using fMRI data that have already been collected.
It was remarkable that LC, ME and HC had roughly parallel time courses for Stroop, practice, surprise and other response patterns that were derived from differences in Rt. The overall similarity suggested that similar cognitive processes were utilized in all subjects. Viable theories of cognition must explain the practice effect for the Incongruent Stimulus and persistent incongruent prestimulus effect that were maintained in all groups and that were not altered by the disease pathologies that caused the distinct global slowing of responses times in LC and ME. Neural mechanisms and network areas recruited to complete the task may be similar in LC, ME and HC.
The strong congruency sequence effect of the incongruent prestimulus suggested prestimulus priming by its distracting and cognitively challenging presentation leading to increased attentional control then followed by recruitment of anticipatory brain activities that continued to be utilized during the subsequent stimulus54. The cognitive processes responsible for the effects continue to be debated55,56but neurological imaging methods have begun to chart the network processes invoked by the Stroop task. Event-related potentials reveal high temporal resolution stages of stimulus processing, semantic level analysis, and response selection in a frontal parietal network49. The dorsolateral prefrontal cortex (DLPFC) is consistently involved for sustained and selective attention during task execution. Anodal transcutaneous direct current stimulation over the DLPFC improves performance on the Stroop task57. The lateral prefrontal cortex is proposed to exert differential control to upregulate Stroop task relevant colour processing and downregulate automatic word reading that is not relevant to the task and instead generates interference58. The posterior DLPFC creates the appropriate rules for the brain to accomplish the current goal and to counteract biases, distractions and irrelevant information59. Efficiency at the Stroop task requires activating brain areas involved in color perception, but not those involved in word encoding or the semantic perception of the word60. The mid-dorsolateral prefrontal cortex selects the goal oriented information such as ink color and excludes irrelevant information such as word meaning59. Activation of the left DLPFC during a Stroop task is related to expectations about the conflicting nature of presentation rather than the conflict itself. The right dorsolateral prefrontal cortex may reduce the attentional conflict or may not be activated until after the conflict is over61.
Medial prefrontal regions, and in particular the anterior cingulate cortex (ACC), are transiently activated during conflict as part of an error prevention network that facilitates corrective actions42. The ACC may monitor bilateral DLPFC function and becomes activated during conflict and interference. This does not appear to be a strategic action to control the Stroop responses42. The ACC helps select an appropriate response and allocates attentional resources62. The anterior dorsal ACC has a predominant role for error checking to determine if the answer is correct or incorrect59. Activity in this region increases when the probability of an error is higher63. The posterior dorsal ACC may activate the medial supplementary motor area leading to the ultimate button press that marks the end of each test61.
DLPFC regions coordinate with posterior parietal regions of the executive control network for visuospatial attention and task switching to ensure the selection of the relevant word colour information and suppression of word reading61. Event-related potentials during the Stroop task demonstrate that presentation of the incongruent stimulus can recruit larger anticipatory resources in premotor and parietal areas than the congruent stimulus but without further activation of frontal areas64. Difficult tasks such as the Incongruent Stimulus may lead to prolonged downstream activation of the left intraparietal lobe and bilateral extrastriate visual cortex that lasts into the next stimulus64. The distracting stimulus may also stimulate the right ventral attention network with activation of the right temporal parietal junction, inferior frontal gyrus and insula65,66.
The right anterior insula is closely connected to the dorsal ACC within the salience network and may recognize the interference of the incongruent condition and recruit inhibitory control mechanisms through the dACC, supplemental motor area (SMA) and separate connections to the right putamen for inhibitory control and movement planning for flexible goal-oriented, adaptive behavior67,68. In this way the incongruent prestimulus may cause prolonged task oversight, conflict recognition (interference) and resolution with the additional steps adding to the longer (slower) Rt.
Practice effects were evident from the power curves and acceleration of Rt values in the Stroop time series. Task repetition (practice) appears to modify brain activation patterns of distinct regions depending on the nature of the conflict being tested with attention networks of the DLPFC and posterior parietal cortex executive control network, ACC and occipital cortex69being implicated in semantic and response conflicts70. Semantic conflicts affect response control regions in the inferior frontal junction, inferior frontal gyrus, insula and pre-supplementary motor areas. The posterior parietal cortex is involved in response conflict. Practice effects for semantic conflict may involve automation of stimulus processing, conflict and response control while practice may increase the extent of cognitive resources recruited to resolve response conflicts. Left hemisphere attention networks may be involved during the early practice stage for semantic conflict but later for response conflict. This type of time dependent practice effect was suggested by the evolution of Rt, surprise and Stroop effects.
Errors lead to slowing of Rt (Rabbitt effect)71. The salience network and temporoparietal junction are activated during error processing to ensure correct responses72 Dorsal posterior cingulate cortex evaluates feedback from erroneous responses. The left superior frontal gyrus mediates post-error behavioral adaptation (prolonged Rt).
These network processes suggest the cascade of control hypothesis where various brain regions act in series to coordinate the multistage network of task processing, conflict identification, resolution and motor activation58. Parallel processes may also compete for task completion43.
The randomization procedure for order of stimuli was a limitation. The scheme was set to generate slightly more incongruent prestimulus Incongruent Stimulus pairings as cognitive challenges for ME and LC subjects in the fMRI studies. However, the scheme did not generate pairings of neutral stimuli in the initial 100s and this required an adjustment for power curve analysis. There was a relatively small number of cC pairings in this period but no adjustments were necessary. The design did not generate runs of purposefully incongruent or congruent stimuli and so Stroop effects were not as large as other studies. Despite this limitation, the derived Rt data will now be used to plan re-analysis of the fMRI data to investigate Stroop and incongruent prestimulus effects and sequential timing of activation for conflict recognition and resolution. Rt and accuracy data were not analyzed separately for each individual but rather were pooled for each group. Post-error slowing was not evaluated73. Sample sizes were different between subject groups in the two cohorts. The first cohort completed 120 tests while the second had two runs of 55 tests separated by a 90 s rest period. Practice effects were maintained over the short break. The order of each combination of word and color were not extracted and so “recency” of episodic retrieval (repetition of scoring responses based on the most recently viewed instance of the same word color combination)74and context dependent retrieval of control states75were not assessed. ME subjects were diagnosed using Fukuda2and International Consensus Criteria3in the first cohort and the latter for the second cohort. Univariate analysis showed the criterion variable did not impact the Rt data and so ME outcomes were pooled for the comparison against LC. The data from the first half of the task had the largest incremental changes in Rt due to practice effects while the second half maintained the relatively steady Rt. Differences in fMRI findings between the first and second halves may identify alterations caused by practice. Excel power equations did not generate asymptotes and so do not provide the minimum time for stimulus assimilation and response76.
Methods
Subjects
The study was approved by Griffith University Human Research Ethics Committee (2022/666) and written informed consent was obtained from all individuals. This cross-sectional investigation was conducted at the National Centre for Neuroimmunology and Emerging Diseases (NCNED) on the Gold Coast, Queensland, Australia. Eligible participants were medical-practitioner referred and assessed using validated Centers for Disease Control and Prevention questionnaire and the NCNED research questionnaires for fatigue affected subjects to record the severities of post-exertional malaise, cognitive disturbances, immune manifestations, thermoregulatory complaints, gastrointestinal symptoms, urinary frequency, body pain, and sleep disturbances. Long-COVID (LC) participants were selected if they had one or more of these symptoms, moderate or worse severity, onset less than 3 months following COVID-19 infection and persistence for at least 3 months according to the WHO working case definition7. ME/CFS (ME) patients met International Case Criteria (ICC)3or 1994 Center for Disease Control (“Fukuda”) criteria2. Cases were reviewed by a clinician experienced in LC and ME/CFS. Healthy controls reported no chronic health condition or evidence of underlying illness. Medical history was requested to identify comorbid manifestations and exclusionary diagnoses including mental illness, malignancies, autoimmune, neurological, or cardiovascular diseases. Female participants were not pregnant or breastfeeding. The study was performed in accordance with the Declaration of Helsinki.
Stroop colour-word task performance data were available for cohorts of healthy controls (HC), ME and LC who had participated in functional magnetic resonance imaging trials (Table 1). The 2016 cohort of HC and ME viewed a continuous string of 120 stimuli over 900s. The 2023 cohort of HC, ME and LC were tested with two runs of 450 s (55 stimuli each) separated by 90s. The average time per stimulus was 7.5s. The two Stroop protocols were dictated by the purposes of the fMRI studies39,77–81. Univariate analysis showed that the HC and ME data from the two cohorts were equivalent and so their data were pooled. The two runs in the 2023 cohort were compared and showed that the 90s interruption did not affect Rt indicating no loss of practice or conflict adaptation effects.
The Stroop colour-word task engages attention, conflict recognition (surveillance), conflict resolution, decision making and motor responses. Each Stroop stimulus displayed two words on a computer screen (Fig. 1). The upper word, either RED, BLUE, YELLOW, GREEN or XXXX, was coloured either red, blue, or yellow or green on a black background. The lower word was either RED, BLUE, YELLOW or GREEN coloured white on a black background. The subject was asked to respond to the question “Does the colour of the upper word agree with the meaning of the lower word?” by pressing one of two buttons on a handpiece to respond ‘yes’ or ‘no’. The Stroop task involved three trial types: incongruent (the upper word is written in different colour from its meaning), congruent (upper word is written in the colour of its meaning) or neutral (upper word is XXXX in a colour that sometimes differed from the meaning of the lower word). The incongruent task is more challenging because of the need to inhibit the automatic impulse to read the top word rather than inspect its colour and the conflict between the upper word meaning vs. color and lower word meaning.
The Stroop stimuli were presented in random order. Proportion contingency effects43,50were fixed by having 30% Congruent, 36% Incongruent and 34% Neutral Stimuli on average for all subjects (total 15,354 stimuli). Using three stimuli meant it was difficult for subjects to develop strategies to predict the next stimulus. This was anticipated to reduce congruency sequence or Gratton effects41. As a result, the Stroop effect of word reading interference on color naming was predicted to be relatively low. Episodic retrieval of recent responses to stimuli were not accessible from the data82.
Stroop task records were scanned to tabulate stimulus onset time, response times (Rt), stimulus type, and button box response for accuracy (fraction of total stimuli). Data were extracted for the nine prestimulus – stimulus pairings from all subjects. Each pairing is reported here with prestimulus in lower case (n, c, i) and Stimulus in upper case (N, C, I) to give pairings such as iN and cC. Response time recorded the time from first observing a new stimulus to pushing the response button. Effects of time were determined as time from start of the task and number of stimuli tested (duration 7.5s per stimulus).
Factors affecting Rt were assessed by univariate general linear model for simple main fixed effects with Sidak correction for multiple comparisons in SPSS version 29 (IBM https://www.ibm.com/products/spss-statistics). Rt was the dependent variable. Independent variables (fixed factors) were disease group (HC, LC, ME), prestimulus (n, c, i), and Stimulus (N, C, I) with time as a covariate. Estimated marginal means were calculated for group, prestimulus, Stimulus and the cross-products after correcting for the effects of time. To assess longitudinal practice effects, the data were allocated to 6 bins and the univariate analysis repeated for log Rt as the dependent variable and log duration as a covariate.
Practice or automaticity effects were determined by power function analysis of Rt vs. stimulation time data43 in Excel. The constant represented the extrapolated y-intercept for Rt at time 0s. The asymptotes were 0 by this method. The absolute value of the exponent was the rate of learning and represented the improvement in Rt as a function of T. Values were compared between HC, LC and ME for each pairing. There was an anomaly with nN due to the randomization program that added more of the Incongruent Stimuli so that no nN pairings were recording in the first 100s. Therefore, nN power curves were corrected by re-aligning the data to 100s.
Variability throughout the trial was assessed from the standard deviations of Rt for equally large groups of consecutive stimuli. It is postulated that SD of Rt should decrease in later segments of the curve compared to the initial period43.
Supplementary Information
Acknowledgements
We are thankful to Ms. Tania Manning, Ms. Kay Schwarz and Ms. Vivienne Baraniuk for editorial support and arranging the recruitment of patients and healthy control participants who donated their time and effort to participate in this study.
Author contributions
JB performed analysis, interpretation of data, drafting, and final approval. KT performed conception, design, data acquisition and final approval. MI performed data acquisition, reviewing, and final approval. ZS performed design, data acquisition, and final approval. LB performed the original conception work, design, analysis, interpretation of data, drafting, and final approval. All authors reviewed the manuscript.
Funding
The Stafford Fox Medical Research Foundation.
Data availability
Data AvailabilityAll data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Holmes, G. P. et al. Chronic fatigue syndrome: a working case definition. Ann. Intern. Med. 108 (3), 387–389 (1988). [DOI] [PubMed] [Google Scholar]
- 2.Fukuda, K. et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International chronic fatigue syndrome Study Group. Ann. Intern. Med. 121 (12), 953–959 (1994). [DOI] [PubMed] [Google Scholar]
- 3.Carruthers, B. M. et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 270 (4), 327–338 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Redefining an Illness [Internet]. [cited 2022 Nov 17]. (2015). https://nap.nationalacademies.org/read/19012/chapter/1
- 5.Carruthers, B. M. Definitions and aetiology of myalgic encephalomyelitis: how the Canadian consensus clinical definition of myalgic encephalomyelitis works. J. Clin. Pathol. 60 (2), 117–119 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thaweethai, T. et al. Development of a definition of Postacute Sequelae of SARS-CoV-2 infection. JAMA (2023). [DOI] [PMC free article] [PubMed]
- 7.Soriano, J. B. et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 22 (4), e102–e107 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long-Term Health Effects of COVID-19 National Academies of Sciences, Engineering, and Medicine. Long-Term Health Effects of COVID-19: Disability and Function Following SARS-CoV-2 Infection. Washington, DC: National Acadamies of Science, Engineering and Medicine; 1–242, (2024). [PubMed]
- 9.Aoun Sebaiti, M. et al. Systematic review and meta-analysis of cognitive impairment in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Sci. Rep. 12 (1), 2157 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceban, F. et al. Fatigue and cognitive impairment in Post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav. Immun. 101, 93–135 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrucci, R. et al. Long-lasting cognitive abnormalities after COVID-19. Brain Sci. 11 (2), 235. 10.3390/brainsci11020235 (2021). [DOI] [PMC free article] [PubMed]
- 12.Azcue, N. et al. Brain fog of post-COVID-19 condition and chronic fatigue syndrome, same medical disorder? J. Transl Med. 20 (1), 569 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muccioli, L. et al. Cognitive and functional connectivity impairment in post-COVID-19 olfactory dysfunction. Neuroimage Clin. 38, 103410 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, Z., Zhang, Z., Zhang, Z., Wang, Z. & Li, H. Cognitive impairment after long COVID-19: current evidence and perspectives. Front. Neurol. 14, 1239182 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capuron, L. et al. Cognitive dysfunction relates to subjective report of mental fatigue in patients with chronic fatigue syndrome. Neuropsychopharmacology. 31 (8), 1777–1784 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Claypoole, K. H. et al. A twin study of cognitive function in chronic fatigue syndrome: the effects of sudden illness onset. Neuropsychology. 21 (4), 507–513 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Cockshell, S. J. & Mathias, J. L. Cognitive deficits in chronic fatigue syndrome and their relationship to psychological status, symptomatology, and everyday functioning. Neuropsychology. 27 (2), 230–242 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Fiedler, N., Kipen, H. M., DeLuca, J., Kelly-McNeil, K. & Natelson, B. A controlled comparison of multiple chemical sensitivities and chronic fatigue syndrome. Psychosom. Med. 58 (1), 38–49 (1996). [DOI] [PubMed] [Google Scholar]
- 19.Lawrie, S. M., MacHale, S. M., Cavanagh, J. T., O’Carroll, R. E. & Goodwin, G. M. The difference in patterns of motor and cognitive function in chronic fatigue syndrome and severe depressive illness. Psychol. Med. 30 (2), 433–442 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Mahurin, R. K. et al. Cognitive processing in monozygotic twins discordant for chronic fatigue syndrome. Neuropsychology. 18 (2), 232–239 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Majer, M. et al. Neuropsychological performance in persons with chronic fatigue syndrome: results from a population-based study. Psychosom. Med. 70 (7), 829–836 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Marcel, B., Komaroff, A. L., Fagioli, L. R., Kornish, R. J. & Albert, M. S. Cognitive deficits in patients with chronic fatigue syndrome. Biol. Psychiatry. 40 (6), 535–541 (1996). [DOI] [PubMed] [Google Scholar]
- 23.Marshall, P. S., Forstot, M., Callies, A., Peterson, P. K. & Schenck, C. H. Cognitive slowing and working memory difficulties in chronic fatigue syndrome. Psychosom. Med. 59 (1), 58–66 (1997). [DOI] [PubMed] [Google Scholar]
- 24.Marshall, P. S. et al. An assessment of cognitive function and mood in chronic fatigue syndrome. Biol. Psychiatry. 39 (3), 199–206 (1996). [DOI] [PubMed] [Google Scholar]
- 25.Michiels, V., de Gucht, V., Cluydts, R. & Fischler, B. Attention and information processing efficiency in patients with chronic fatigue syndrome. J. Clin. Exp. Neuropsychol. 21 (5), 709–729 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Smith, A. P., Behan, P. O., Bell, W., Millar, K. & Bakheit, M. Behavioural problems associated with the chronic fatigue syndrome. Br. J. Psychol. 84 (Pt 3), 411–423 (1993). [DOI] [PubMed] [Google Scholar]
- 27.Michiels, V., Cluydts, R. & Fischler, B. Attention and verbal learning in patients with chronic fatigue syndrome. J. Int. Neuropsychol. Soc. 4 (5), 456–466 (1998). [DOI] [PubMed] [Google Scholar]
- 28.Stroop, J. R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 18 (6), 643–662 (1935). [Google Scholar]
- 29.MacLeod, C. M. Half a century of research on the Stroop effect: an integrative review. Psychol. Bull. 109 (2), 163–203 (1991). [DOI] [PubMed] [Google Scholar]
- 30.Leung, H. C., Skudlarski, P., Gatenby, J. C., Peterson, B. S. & Gore, J. C. An event-related functional MRI study of the stroop color word interference task. Cereb. Cortex. 10 (6), 552–560 (2000). [DOI] [PubMed] [Google Scholar]
- 31.Periáñez, J. A., Lubrini, G., García-Gutiérrez, A. & Ríos-Lago, M. Construct validity of the Stroop Color-Word Test: influence of speed of visual search, Verbal Fluency, Working Memory, Cognitive Flexibility, and conflict monitoring. Arch. Clin. Neuropsychol. 36 (1), 99–111 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Cattell, J. M. The time it takes to see and name objects. Mindos-XI (41), 63–5 (1886). [Google Scholar]
- 33.Beaumont, A. et al. Reduced cardiac vagal modulation impacts on cognitive performance in chronic fatigue syndrome. PLoS One. 7 (11), e49518 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiClementi, J. D., Schmaling, K. B. & Jones, J. F. Information processing in chronic fatigue syndrome: a preliminary investigation of suggestibility. J. Psychosom. Res. 51 (5), 679–686 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Ray, C., Phillips, L. & Weir, W. R. Quality of attention in chronic fatigue syndrome: subjective reports of everyday attention and cognitive difficulty, and performance on tasks of focused attention. Br. J. Clin. Psychol. 32 (3), 357–364 (1993). [DOI] [PubMed] [Google Scholar]
- 36.Robinson, L. J. et al. Impairments in cognitive performance in chronic fatigue syndrome are common, not related to co-morbid depression but do associate with autonomic dysfunction. PLoS One. 14 (2), e0210394 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ortelli, P. et al. Global slowness and increased intra-individual variability are key features of attentional deficits and cognitive fluctuations in post COVID-19 patients. Sci. Rep. 12 (1), 13123 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin, E. M. et al. Persistent cognitive slowing in post-COVID patients: longitudinal study over 6 months. J. Neurol. 271 (1), 46–58 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnden, L., Thapaliya, K., Eaton-Fitch, N., Barth, M. & Marshall-Gradisnik, S. Altered brain connectivity in Long Covid during cognitive exertion: a pilot study. Front. Neurosci. 17, 1182607 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egner, T. & Hirsch, J. The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage. 24 (2), 539–547 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Gratton, G., Coles, M. G. & Donchin, E. Optimizing the use of information: strategic control of activation of responses. J. Exp. Psychol. Gen. 121 (4), 480–506 (1992). [DOI] [PubMed] [Google Scholar]
- 42.Carter, C. S. et al. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc. Natl. Acad. Sci. U S A. 97 (4), 1944–1948 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Logan, G. D. Toward an instance theory of automatization. Psychol. Rev. 95 (4), 492–527 (1988). [Google Scholar]
- 44.Martinon, L. M. et al. Distributional analyses reveal the polymorphic nature of the Stroop interference effect: it’s about (response) time. Mem. Cognit. 52 (6), 1229–1245. 10.3758/s13421-024-01538-3 (2024). [DOI] [PubMed]
- 45.Heathcote, A., Brown, S. & Mewhort, D. J. The power law repealed: the case for an exponential law of practice. Psychon Bull. Rev. 7 (2), 185–207 (2000). [DOI] [PubMed] [Google Scholar]
- 46.Parris, B. A., Hasshim, N., Wadsley, M., Augustinova, M. & Ferrand, L. The loci of Stroop effects: a critical review of methods and evidence for levels of processing contributing to color-word Stroop effects and the implications for the loci of attentional selection. Psychol. Res. 86 (4), 1029–1053 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Algom, D. & Chajut, E. Reclaiming the Stroop Effect back from control to Input-Driven attention and perception. Front. Psychol. 10, 1683 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caldwell, J. & Vahidsafa, A. https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Quantifying_Nature/Significant_Digits/Propagation_of_Error. Accessed 29 Aug 2023.
- 49.Kalanthroff, E. & Henik, A. Preparation time modulates pro-active control and enhances task conflict in task switching. Psychol. Res. 78 (2), 276–288 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Schmidt, J. R. & Besner, D. The Stroop effect: why proportion congruent has nothing to do with congruency and everything to do with contingency. J. Exp. Psychol. Learn. Mem. Cogn. 34 (3), 514–523 (2008). [DOI] [PubMed] [Google Scholar]
- 51.Sasso, E. M. et al. Transient receptor potential melastatin 3 dysfunction in post COVID-19 condition and myalgic encephalomyelitis/chronic fatigue syndrome patients. Mol. Med. 28 (1), 98 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thapaliya, K., Marshall-Gradisnik, S., Staines, D. & Barnden, L. Diffusion tensor imaging reveals neuronal microstructural changes in myalgic encephalomyelitis/chronic fatigue syndrome. Eur. J. Neurosci. 54 (6), 6214–6228 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bispo, D. D. et al. Brain microstructural changes and fatigue after COVID-19. Front. Neurol. 13, 1029302 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duthoo, W., Abrahamse, E. L., Braem, S., Boehler, C. N. & Notebaert, W. The Congruency sequence effect 3.0: a critical test of conflict adaptation. PLoS One. 9 (10), e110462 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duthoo, W., Abrahamse, E. L., Braem, S., Boehler, C. N. & Notebaert, W. The heterogeneous world of congruency sequence effects: an update. Front. Psychol. 5, 1001 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Egner, T. Congruency sequence effects and cognitive control. Cogn. Affect. Behav. Neurosci. 7 (4), 380–390 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Perrotta, D., Bianco, V., Berchicci, M., Quinzi, F. & Perri, R. L. Anodal tDCS over the dorsolateral prefrontal cortex reduces Stroop errors. A comparison of different tasks and designs. Behav. Brain. Res. 405, 113215 (2021). [DOI] [PubMed] [Google Scholar]
- 58.Banich, M. T. The Stroop Effect occurs at multiple points along a Cascade of Control: evidence from cognitive neuroscience approaches. Front. Psychol. 10, 2164 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Banich, M. T. et al. fMri studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. J. Cogn. Neurosci. 12 (6), 988–1000 (2000). [DOI] [PubMed] [Google Scholar]
- 60.Bush, G. et al. The counting Stroop: an interference task specialized for functional neuroimaging–validation study with functional MRI. Hum. Brain Mapp. 6 (4), 270–282 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Milham, M. P., Banich, M. T., Claus, E. D. & Cohen, N. J. Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. Neuroimage. 18 (2), 483–493 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Pardo, J. V., Pardo, P. J., Janer, K. W. & Raichle, M. E. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc. Natl. Acad. Sci. U S A. 87 (1), 256–259 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gruber, S. A., Rogowska, J., Holcomb, P., Soraci, S. & Yurgelun-Todd, D. Stroop performance in normal control subjects: an fMRI study. Neuroimage. 16 (2), 349–360 (2002). [DOI] [PubMed] [Google Scholar]
- 64.Bianco, V. et al. Electrophysiological evidence of anticipatory cognitive control in the stroop task. Brain Sci. 11 (6), 783. 10.3390/brainsci11060783 (2021). [DOI] [PMC free article] [PubMed]
- 65.Ghahremani, A., Rastogi, A. & Lam, S. The role of right anterior insula and salience processing in inhibitory control. J. Neurosci. 35 (8), 3291–3292 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aron, A. R., Robbins, T. W. & Poldrack, R. A. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn. Sci. 18 (4), 177–185 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Bari, A. & Robbins, T. W. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 108, 44–79 (2013). [DOI] [PubMed] [Google Scholar]
- 68.Deng, Y., Wang, X., Wang, Y. & Zhou, C. Neural correlates of interference resolution in the multi-source interference task: a meta-analysis of functional neuroimaging studies. Behav. Brain Funct. 14 (1), 8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ioannucci, S. et al. Neural fatigue by passive induction: repeated stimulus exposure results in cognitive fatigue and altered representations in task-relevant networks. Commun. Biol. 6 (1), 142 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen, Z., Lei, X., Ding, C., Li, H. & Chen, A. The neural mechanisms of semantic and response conflicts: an fMRI study of practice-related effects in the Stroop task. Neuroimage. 66, 577–584 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Rabbitt, P. M. Errors and error correction in choice-response tasks. J. Exp. Psychol. 71 (2), 264–272 (1966). [DOI] [PubMed] [Google Scholar]
- 72.Cieslik, E. C., Ullsperger, M., Gell, M., Eickhoff, S. B. & Langner, R. Success versus failure in cognitive control: Meta-analytic evidence from neuroimaging studies on error processing. Neurosci. Biobehav Rev. 156, 105468 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pfister, R. & Foerster, A. How to measure post-error slowing: the case of pre-error speeding. Behav. Res. Methods. 54 (1), 435–443 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Giesen, C. G., Schmidt, J. R. & Rothermund, K. The Law of Recency: an episodic stimulus-response Retrieval Account of habit Acquisition. Front. Psychol. 10, 2927 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dignath, D., Johannsen, L., Hommel, B. & Kiesel, A. Reconciling cognitive-control and episodic-retrieval accounts of sequential conflict modulation: binding of control-states into event-files. J. Exp. Psychol. Hum. Percept. Perform. 45 (9), 1265–1270 (2019). [DOI] [PubMed] [Google Scholar]
- 76.Newell, A. & Rosenbloom, P. Mechanisms of skill acquisition and the law of practice. 1–52 p. (1980).
- 77.Barnden, L. R. et al. Intra brainstem connectivity is impaired in chronic fatigue syndrome. Neuroimage Clin. 24, 102045 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Su, J., Thapaliya, K., Eaton-Fitch, N., Marshall-Gradisnik, S. & Barnden, L. Connectivity between salience and default Mode Networks and Subcortical Nodes distinguishes between two classes of myalgic Encephalomyelitis/Chronic fatigue syndrome. Brain Connect. 13 (3), 164–173 (2023). [DOI] [PubMed] [Google Scholar]
- 79.Shan, Z. Y. et al. Neuroimaging characteristics of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a systematic review. J. Transl Med. 18 (1), 335 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Inderyas, M., Thapaliya, K., Marshall-Gradisnik, S., Barth, M. & Barnden, L. Subcortical and default mode network connectivity is impaired in myalgic encephalomyelitis/chronic fatigue syndrome. Front. Neurosci. 17, 1318094 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barnden, L. et al. Anti-correlated myelin-sensitive MRI levels in humans consistent with a subcortical to Sensorimotor Regulatory process-multi-cohort multi-modal evidence. Brain Sci. 12 (12), 1693. 10.3390/brainsci12121693 (2022). [DOI] [PMC free article] [PubMed]
- 82.Rothermund, K., Gollnick, N. & Giesen, C. G. Accounting for Proportion Congruency effects in the Stroop Task in a Confounded Setup: Retrieval of stimulus-response episodes explains it all. J. Cogn. 5 (1), 39 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data AvailabilityAll data generated or analysed during this study are included in this published article and its supplementary information files.